Endometrial Cancers
Anatomy
For a discussion on anatomy and nodal regions please see Cervix.
Epidemiology
Pathology
The majority of cases are endometrioid, which is divided into low, intermediate and high grades (Grade I-III). These are commonly low grade, estrogen responsive, and are much more common. Papillary serous and clear cell cancers are much more agressive and should be considered high risk disease.
More recently, histopathlogists have divided the two groups into "Type I" and "Type II" cancers. Type II are the serous and clear cell cancers, which are generally more agressive and much less common than endometriod cancers.
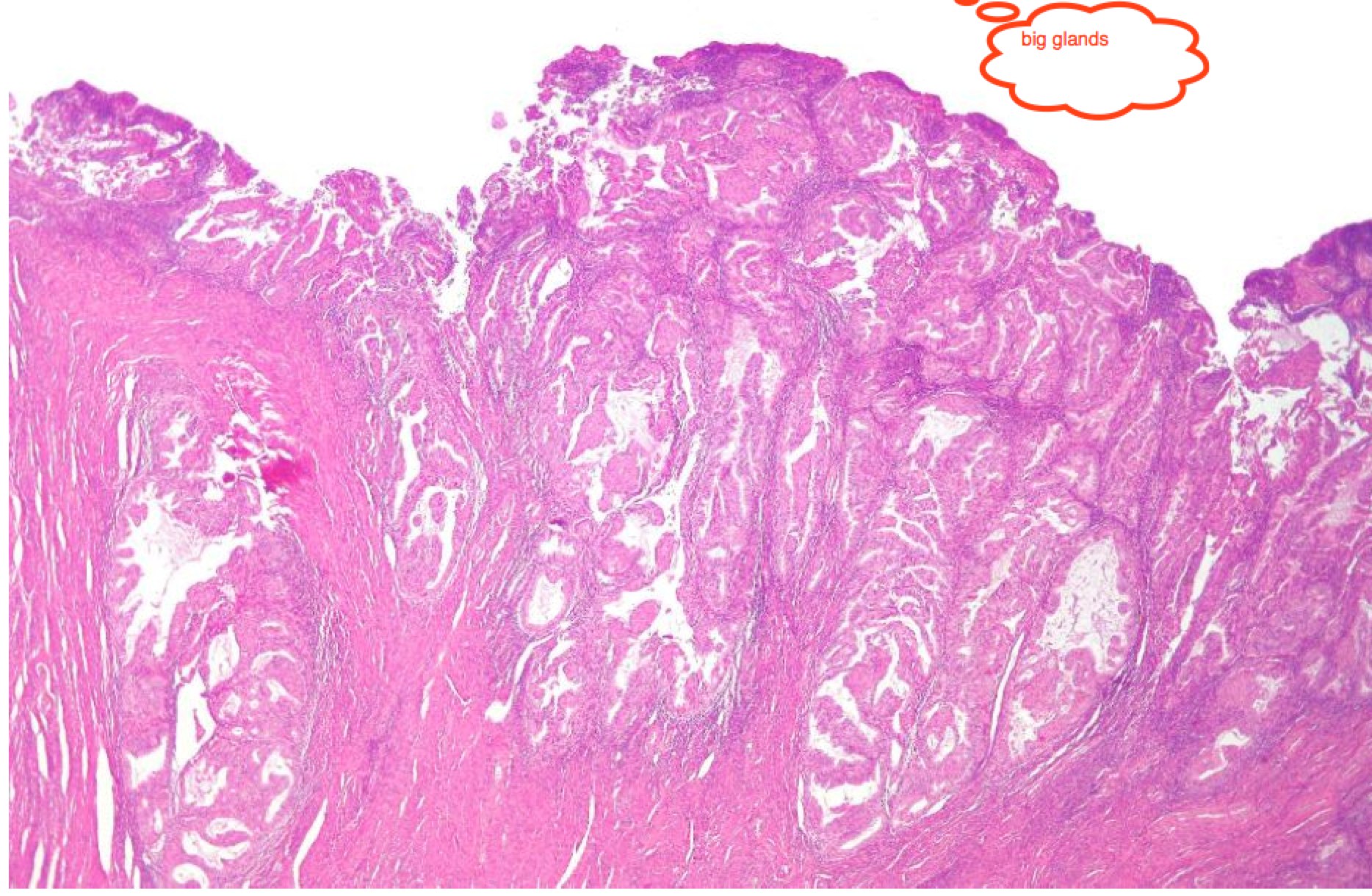 | 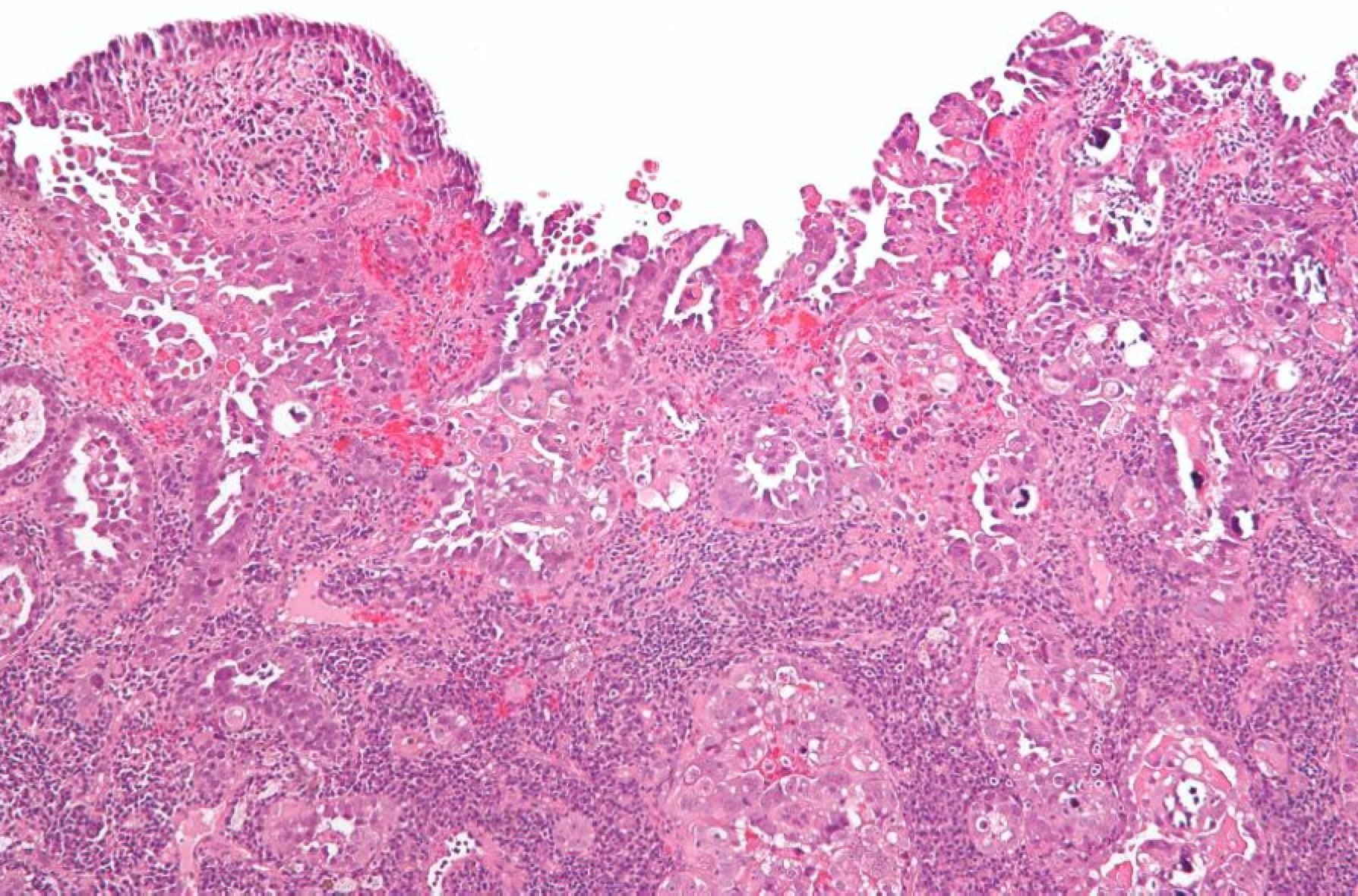 |
| Endometrioid: Big well structured glands | Papillary serous with sheets of cells between disorganized glands |
Staging
Staging has been revised. Older studies divided FIGO stage I into A, B, C for endometrium only, < 50% myometrial invasion and &ge 50% myometrial invasion. The revised Staging reduces this to Stage I A/B for < 50% MMI adn > 50% MMI. Cervical Stromal invasion was formerly staged II A/B for endocervical or stromal invasion. This is now eliminated in favor of Stage II: cervical invasion present. Stage IIIA was revised to eliminate pelvic washing cytology and now includes only adenexa or serosal involvement. Stage IIIB adds parametrial involvement (formerly just vaginal involvement, now both.) Stage IIIC adds a descriptor for pelvic (IIIC1) and Para-aortic nodes (IIIC2).
Natural History
Clinical Workup and Evaluation
Endometrial cancer is usually diagnosed with spotting in post-menopausal women, metrorrhagia, or menometrorrhagia. Tissue diagnosis is the gold standard and can be obtained either through Dilation and Curettage/hysteroscopy or with a trans-cervical endometrial biopsy. Prior to either procedure, inspection of the cervix for signs of cervical cancer and a PAP with ECC should be performed to be certain there is no disease in the cervix. Biopsy contamination can easily obscure an occult cervical cancer leading to mis-diagnosis.
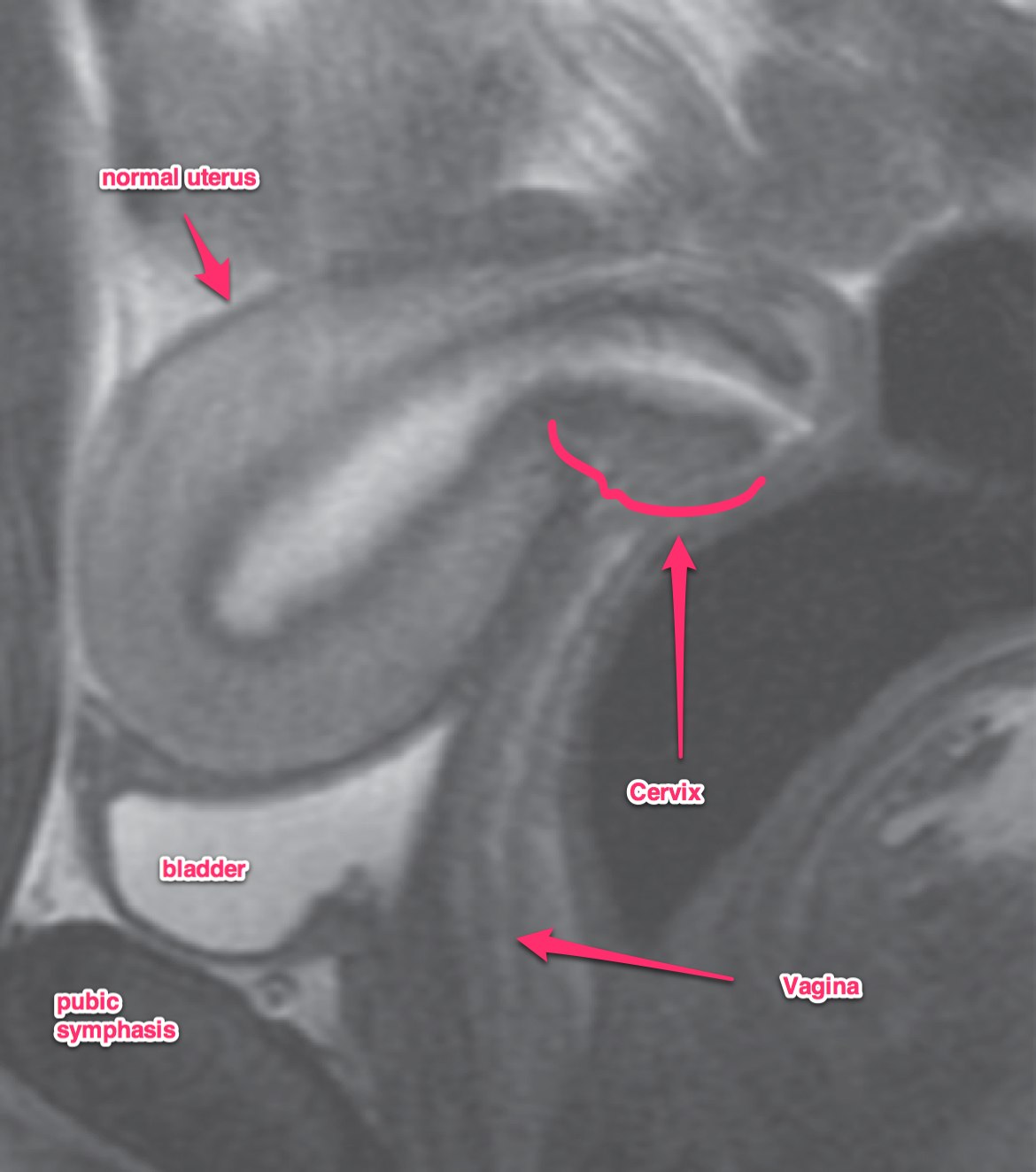
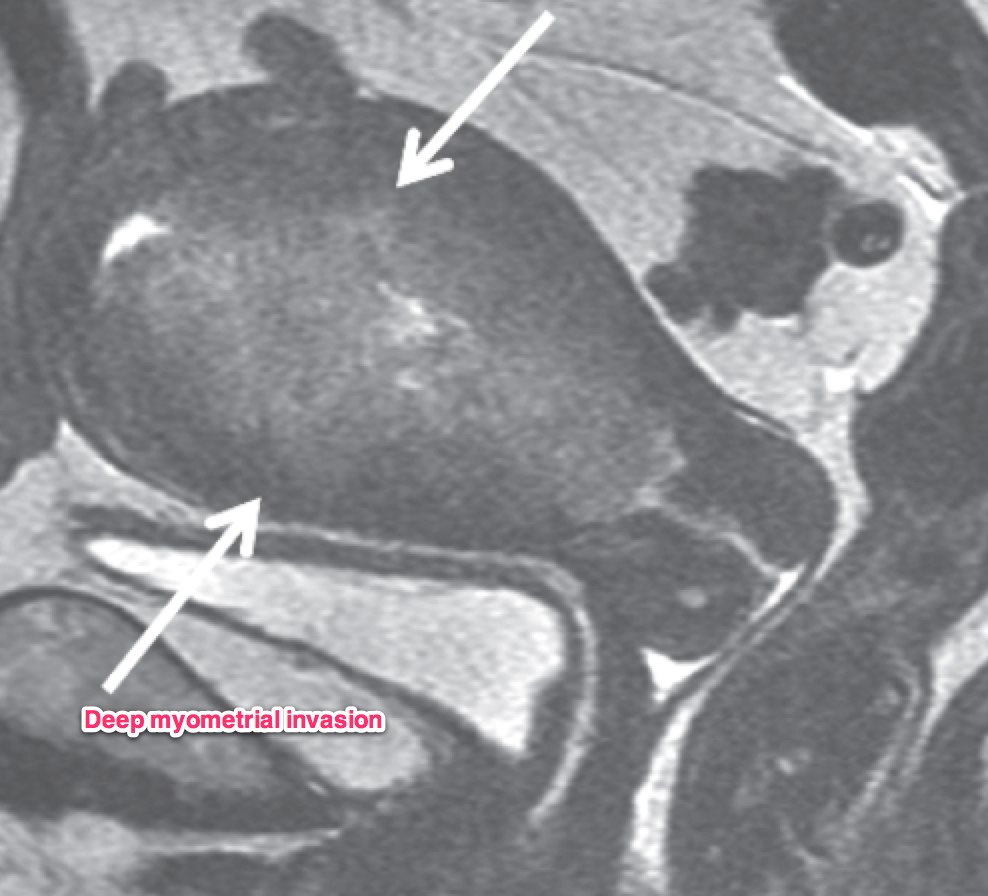
A transvaginal ultrasound should be obtained. In post-menopausal women, the expected endometrial thickness is < 4 mm. In pre-menopausal women, the thickness varies with the menstrual cycle. MRI is useful in identifying suspicious hyperplasia. If a thickened myometrium is visible on contrast enhanced CT, it is likely there is > 50% myometrial invasion. Cervical stromal invasion is seen on CT as an enlarged cervix, > 3 cm, heterogeneous stroma with low attenuation. Parametrial invasion is seen by loss of fat in the periureteral region, and fat plane near the pelvic sidewall.
Dynamic contrast enhanced MRI is optimal for MRI studies with accuracy of 85% or better. If there is a clear junctional zone between the tumor and the myometrium, the disease is most likely limited to the myometrium. MRI can also determine depth of cervical stromal invasion.
 |  |
General Management and Treatment
Surgery
Surgical management is the mainstay of uterine cancer treatement, generally with a total hysterectomy. This is increasingly done using laparascopic assisted vaginal hysterectomy technieques for early uterine cancer. See Cervix for details on the types of hysterectomies commonly used.
For low grade, localized disease, several studies have looked at the role of node dissection. Duke and MRC-UK ASTEC examined the role of PLND. The incidence of positive nodes in low risk disease — Stage IA, Grade 1-2 is 2%. For high-intermediate risk (Stage IB or Grade 3) the risk is 9%. While there was a modest difference in recurrence free survival (p=0.01), there was no difference in overall survival and there was a difference in moderate to severe complications with LND increasing the rate from 12% (no PLND) to 17% (with LND).
From these studies, Chino concludes that LND cannot be routinely recommended, and it will upstage about 10% with pathologic risk factors: IB or Grade 3 disease.
Radiation Therapy
Radiation therapy also has a broad role in the treatment of endometrial cancers. The PORTEC studies examined the role of adjuvant radiation therapy in endometrial cancers. These studies have given us insight to the current recommendations for adjuvant radiation treatment.
PORTEC-1
The PORTEC-1 (Creutzberg, 2000), examined early endometrial cancers IB/Grade 1, and 2, IA Grade 3 (2009 FIGO staging). This study randomized patients to two arms: no further treatment v. whole pelvis radiation therapy.
- Standard Arm:
- No further postoperative treatment
- Test Arm:
- Whole Pelvis radiation therapy to 46 Gy at 2 Gy/fraction.
This trial demonstrated improved local regional recurrence rates with whole pelvic radiation therapy. LRR5 improved from 14% in the observation arm to 4% in the radiation arm. (p<0.001). There was no change in overall survival at around 85%. Vaginal recurrences were most common at 75% in the observation arm and the OS2 after vaginal recurrence was 79%. PORTEC excluded IB Grade 3 patients. (Creutzberg et al. Lancet 2000;355:1404).
An update in the IJROBP in 2005 by Sholten (Scholten et al. IJROBP 2005;63:834-838) found the following:
- LRR10 Radiation: 5%, v. 14% (observation arm)
- OS10 Radiation: 66% v. 73% (observation arm ) p=0.09
- DSS10 Radiation: 11% v. 9% (observation arm) p=0.47
If IA/Grade 1 cases are excluded, then the LRR10 was 5% in the RT arm and the observation arm rose to 17%.
A second PORTEC-1 update by Creutzberg in 2011 (Creutzberg et al. IJROBP 2011;e631-e638) concludes in a 15 year:
- LRR15 Radiation: 6%, v. 15.5% (observation arm)
- OS15 Radiation: 52% v. 54% (observation arm ) p=0.09
- DSS15 Radiation: 14% v. 13% (observation arm) p=0.47
Creutzberg included second malignancies in the treated field and found that 19% occured overall, with 22% in the Radiation arm, and 16% in the observation arm. He notes that survival after recurrence was better in the observation arm, and that most recurrences were in the vagina. Age and High or intermediate risk groups were important prognostic indicators with older age being a worse prognostic factor. The risk of endometrial cancer related death was significantly higher in women 60 and older.
GOG 99: Whole Pelvis Radiation v. Observation in Intermediate Risk Stage IA/B post TAH/BSO with LND
This study examined intermediate risk Stage IA/B and occult Stage II endometrial cancer treated surgically with TAH/BSO and lymph node dissection. Whole Pelvic RT was 50.4 Gy.
- Standard Arm:
- No further postoperative treatment
- Test Arm:
- Whole Pelvis radiation therapy to 50.4 Gy at 1.8 Gy/fraction.
Findings
GOG 99 (Keys) found a local recurrence rate of 12% (observation) v. 3% (whole pelvic RT), but no survival benefit at 86% (obs) v. 92% (WPRT, p=0.56). A subgroup analysis restricted to High intermediate risk groups and stratified by age was performed. Three risk factors were examined: LVSI, Grade 2-3 and deep myometrial invasion. They found a 2 year recurrence rate of 26% in the observation arm and 6% in the radiation arm.
Keys concluded: Older women do worse and need treatment. We should consider adjuvant treatment in high and intermediate risk groups.
PORTEC-2: Vaginal Cuff brachytherapy v. Whole pelvis Radiation therapy
This study looked at IB Grade 1-2 and IA grade 3 disease. IB/Grade 3 was not allowed on the trial. The primary endpoint was vaginal recurrence.
- Standard Arm:
- Whole Pelvis Radiation to 46 Gy at 2 Gy/fraction
- Test Arm:
- Vaginal brachytherapy to 21 Gy at 7 Gy x 3 fractions to d=5mm, covering 1/2 the vaginal length.
- LDR was allowed to a dose of 30 Gy over about 72 hours.
There was little difference in vaginal recurrence between EBRT and VBT, but there was a 3% difference in favor of EBRT in pelvic control. Toxicity was significantly higher in the EBRT arm. Again there was no survival benefit. They conclude that vaginal cuff brachytherapy was the treatment of choice for most intermediate risk women.
Swedish Low Risk Trial of Vaginal Brachytherapy v No Further treatment
This was a trial of low risk patients, Stage IA (inner 1/2) Grade 1-2 disease treated with observation or vaginal cuff brachytherapy.
- Standard Arm:
- No further postoperative treatment
- Test Arm:
- Vaginal Cuff brachytherapy to 3-8 Gy x 3-6 fractions to 5 mm depth
They found vaginal recurrences at 1.2% with brachytherapy and 3.1% without brachytherapy. Grade 1-2 toxicity was higher with treatment at 2.8% v. 06%. They conclude: It is safe to omit Vaginal Brachytherapy in low risk patients.
Swedish Intermediate Risk Trial of Vaginal Brachytherapy ± WPRT
This trial looked at Stage I endometriod adenocarcinoma with at least one risk factor: Grade 3, Stage IB or DNA aneuploidy.
- Standard Arm:
- Vaginal Brachytherapy
- 3.0 Gy x 6 fractions to 18 Gy, or 5.9 Gy x 3 fractions to 17.7 Gy or 20 Gy x 1 fraction (LDR) to the 5 mm dose point.
- Test Arm:
- Whole Pelvis radiation therapy to 46 Gy at 2 Gy/fraction and VBT as above.
The study confirmed a 3% improvement in regional (pelvic) control with 5% in the brachytherapy arm and 1.5% in the WPRT+VBT arm. There was no survival difference. Toxicity was increased in the whole pelvic arm.
GOG 249 Whole pelvis v. VBT → Carbo/taxol x3 cycles.
There was no difference in RFS2yr or OS2yr at 83% and 93% respectively.
PORTEC-3 is looking at early stage high risk cancers ≥ IB/grade 3 disease. PORTEC-4 is looking at a 3 arm trial of obs v. VBT at 5 Gy x 3 to 5 mm, v. VBT at 7 Gy x 3 to 5 mm.
Adjuvant Treatment Recommendations
- Stage IA Grade 1: OBSERVE
- Stage IA Grade 2-3, Stage IB Grade 1-2: Offer VBT in one of the following schemes:
- 7 Gy x 3 = 21 Gy at d=5 mm
- 5 Gy x 5 = 25 Gy at d=5 mm
- 6 Gy x 5 = 30 Gy at surface.
- Stage IB Grade 3: Strongly consider VBT or WPRT. Have the nodes been adequately dissected?
- Stage II (cervix invasion) WPRT + VTB ± chemotherapy, 45 Gy → 4 Gy x 3 = 12 Gy to 5 mm
- Stage III:
- RTOG 9708:
- WPRT/EFRT + VBT + CDDP (50 mg/m2 x 2 cycles)
- 4 cycles of adjuvant CDDP/taxol
- Sequential chemotherapy (carbo/taxol) → radiation, sandwich or otherwise
- RTOG 9708:
| Stage | Grade I | Grade II | Grade III |
|---|---|---|---|
| IA: Inner half | Observe | VC-BT 6 Gy x 5 | VC-BT 6 Gy x 5 |
| IB: Outer half | VC-BT 6 Gy x 5 | VC-BT 6 Gy x 5 | VC-BT 6 Gy x 5 Consider Pelvic RT |
| II: Cervical Invasion | WPRT+VC-BT ± chemotherapy (45 Gy → 4 Gy x 3 at 5mm) | ||
| III: Parametrium/Nodes | WPRT/EFRT + VCBT + CDDP X2 → CDDP/TAXOL X 4 | ||
Radiation Therapy Treatment Planning And Techniques
Techniques and limitations are similar as for post-operative cervical cancer techniques. Please see Cervix: Radiation Therapy Treatment Planning and Techniques
Outcomes, Patterns of Failure, Prognostic Indicators
Adverse Risk Factors
There a several adverse prognostic factors, among these being age, histologic subtype, grade, depth of myometrial invasion, lymphovascular space invasion, involvement of the lower uterine segement and involvement of the cervix, adenexa, and nodes.
Age
Older age is associated with increasing risk, even when treated as agressively as younger counterparts. Age ≥ 60 is predictive of local-regional recurrence and death in Stage IA grade 1/2 desease, with a hazard ratio of 3.9.
Histologic Subtype
Clear cell and papillary serous subtypes do worse. Endometrioid Grade 3 does worse.
- OS5 Endometrioid = 83%
- OS5 Papillary serous = 53%
- OS5 Clear Cell = 63%
For women with Stage III-IV disease, only 14% were endometrioid histology, compared with 42% for serous and 33% for clear cell cancers.
Grade
Tumor grade is a good prognostic indicator. Grade is directly related to the depth of myometrial invasion and nodal metastases.
Side Effects and Complications of Treatment
Lymph Node Dissection (ASTEC)
ASTEC/MRC-UK trial looked the utility of PLND in 1408 women with presumed Stage I disease. This was randomized to two arms:
- TAH/BSO/Peritoneal Washing/PA node palpation
- TAH/BSO/Peritoneal Washing/PA node palpation PLUS Pelvic Node Dissection
In low risk, grade 1-2 Stage IA disease (inner half or endometrium only) 2% had positive nodes. In high risk or intermediate riks (Stage IB or Grade 3) 9% had positive nodes. There was a slight recurrence free survival advantage in the node dissectino arm, but no overall survival advantage and there was significant toxicity. Moderate to severe surgical complicaitons were 12% in no LND, and 17% in PLND. PLND upstages 10% with pathologic risk factors including IB or Grade 3 disease, but is not recommended due to the significantly increased toxicity.