Cervical Cancers
Anatomy
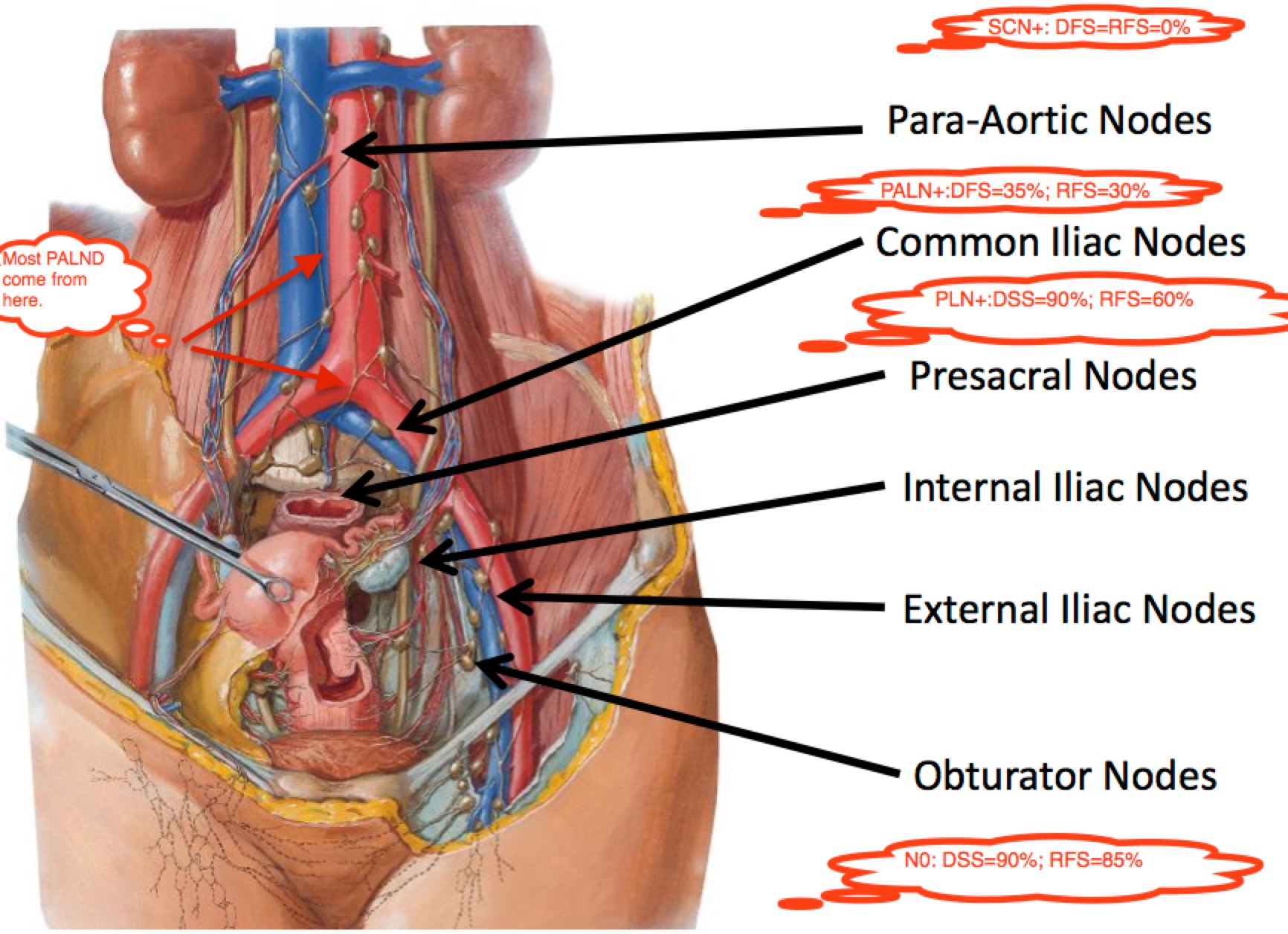
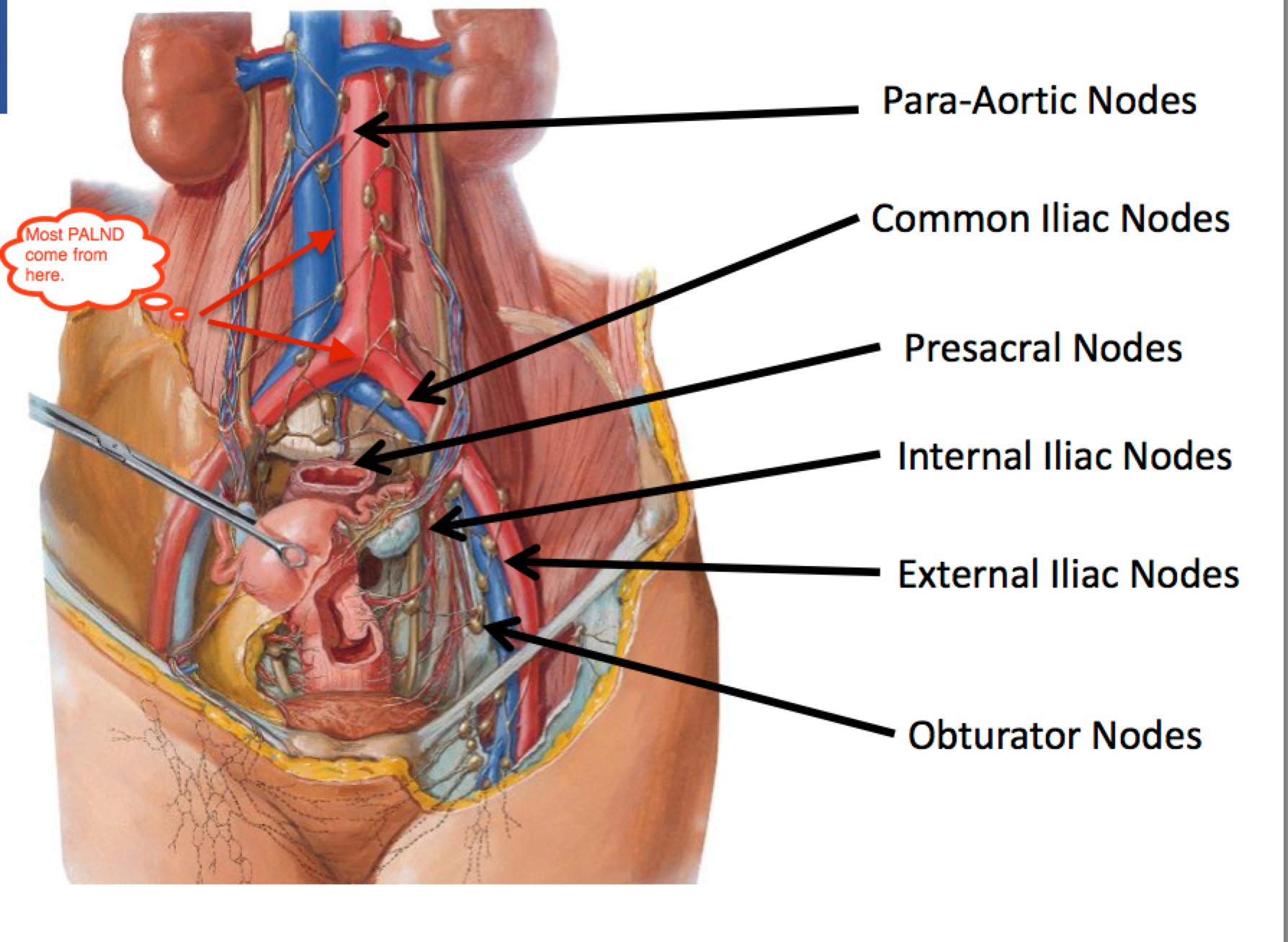
Nodal Anatomy and Regions At Risk
The following identifies the nodal regions at risk of involvement from cervical cancer. FIGO Stage is predictive of node positivity, and Survival is a function of node positivity. FIGO Stage I-III survival ranges between 95% and 65% for node negative disease. For Pelvic only node positive disease, survival drops to 75% → 40%. For PALN positive disease, it drops to between 55% and 30%. If the Supraclavicular nodes are involved, survival is effectively zero.
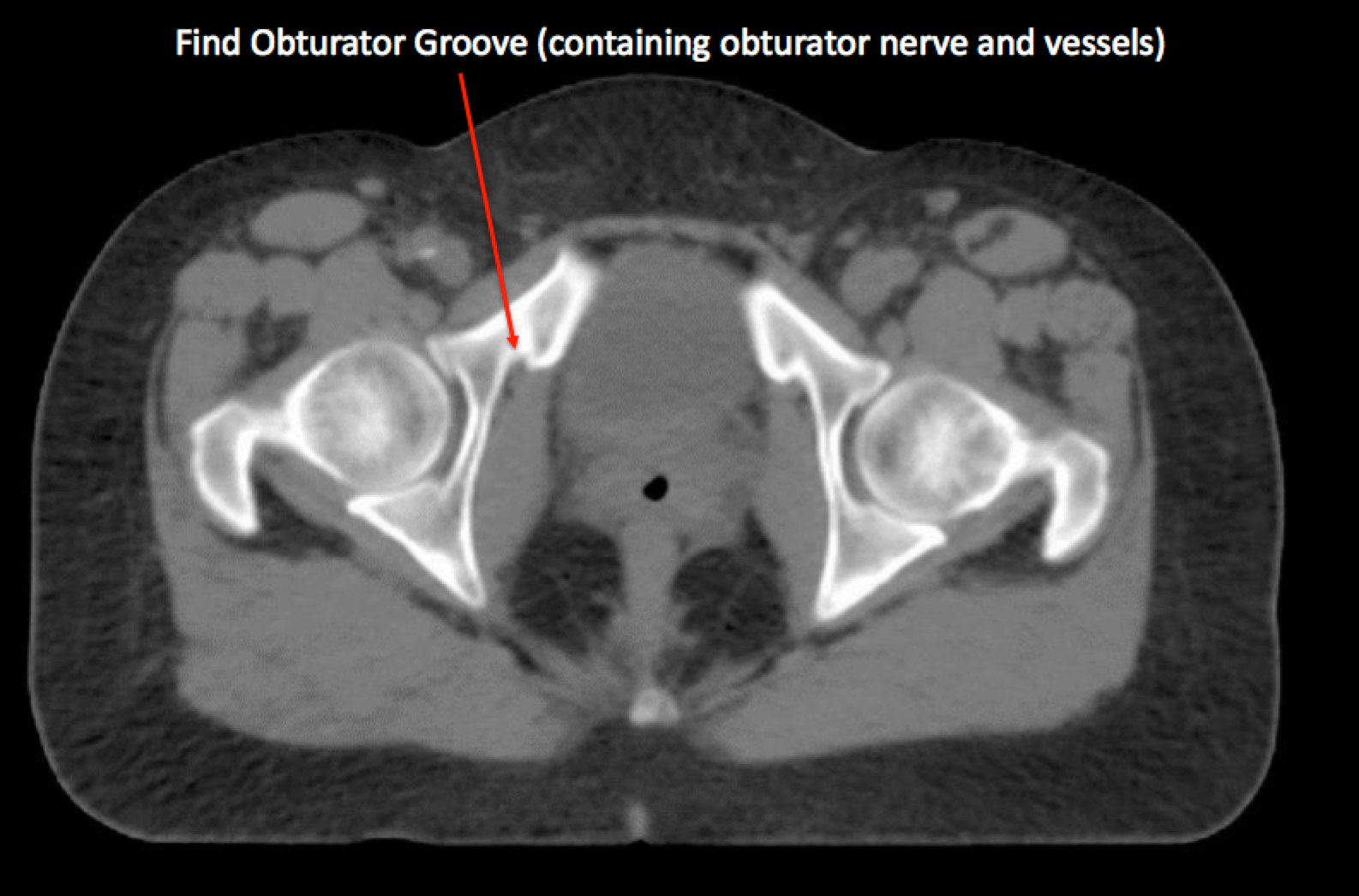
To identify the obturator nodes the following landmarks may be used. (Note: this works for any pelvis where one wishes to find the obturator nodes):
 |
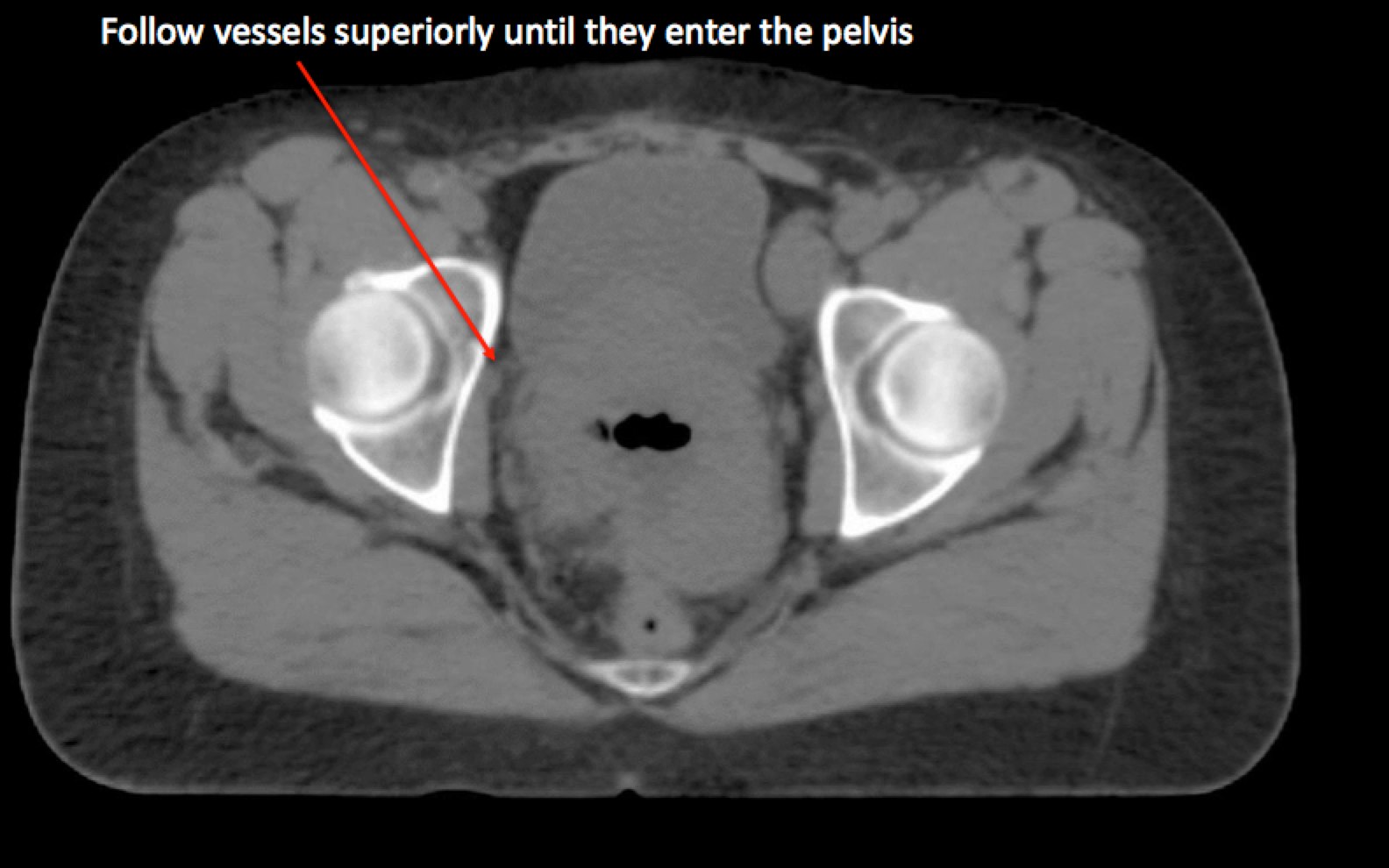 |
Epidemiology
Pathology
Most cervical cancers are squamous cell cancer (approximately 90%). 10% are adenocarcinomas and 1-2% are clear cell. Verrucous carcinoma is a variant of a verwe well differentiated squamous cell carcinoma which tends to occur locally without distant metastases.
Natural History
The most common clinical presentation of cervical cancer is pain or post-coital bleeding. In developed nations, the PAP, followed by colposcopy and biopsy along with HPV testing most commonly detect assymptomatic cervical cancer. There is a continuum of disease from LSIL (low grade squamous intra-epithelial lesion), HSIL (high grade SIL), carcinoma in situ, invasive cervical cancer. Adenocarcinomas also present, though less commonly than squamous cell cancers. On colposcopy, a visible exophytic lesion at the transition zone between the endocervical OS and the cervix are most commonly seen. More advanced disease presents as a larger mass, or a barrel cervix. Patients with more advanced disease may present with inter-menstrual bleeding (metrorrhagia), heavier bleeding (menorhagia) or both (metro-menorrhagia). Bleeding can progress to symptomatic anemia.
More advanced cervical cancer can progress to bowel obstruction, hydronephrosis leading to renal failure, flank pain, rectal bleeding, pelvic sidewall lymphadenopathy causing lower extremety edema. Advanced case can cause foul smelling vaginal discharge, or lumbo-sacral pain with para-aortic lymphatic involvement.
Clinical Workup and Evaluation
The FIGO staging system is independent of modern imaging. FIGO rationalizes this by stating that CT imaging is not available in many areas of the world where cervical cancers is prevalent. While this is true, the world is changing and CT imaging is becaming more prevalent and available. Workup should include CT imaging to assess extent of disease and treatment approach. PET/CT should be considered for bulky IB/IIA and Stage II/III and suspect stage IV disease.
The AJCC staging system requires nodal staging, which FIGO feels is not available in many settings where cervical cancer is prevalent. Where nodal staging is available, it should be used to guide treatment decision making and treatment planning.
A comprehensive pelvic examination should be performed. Supraclavicular (Virchow's) and inguinal nodes should be assessed. Abdomen and liver should be assessed for masses. The pelvic examination should include an inspection of the external genetalia, looking for abnormalities, vaginal discharge, mal-odors. The speculum exam should fully visualize the cervix, vagina, with particular attention to masses on the cervix. Lugol's solution may enhance areas of concern which should be biopsied. (If it's white, take a bite.) Lugols is a stain that identifies areas with lowered staining, reflective of potential cancer. A bimanual exam should be performed to assess for masses, cervical motion tenderness, and ovarian abnormalities. A rectovaginal exam should be performed to assess the parametria and pelvic sidewall involvement. If there is evidence of rectal bleeding, a sigmoidoscopy should be performed to assess the rectum. Likewise, a cysoscopy should be performed to assess the bladder in cases of hematuria. It should be performed in all cases where there are clinical Stage IIB/III/IVA disease. (Disease which has parametrial extension (IIB), Pelvic sidewall involvement or hydronephrosis (IIIB), extension to the lower vagina (IIIA), bladder or rectal invasion (IVA) ).
Where available, (does not contribute to FIGO staging), an MRI of the pelvis is helpful in assessing extent of disease, and may replace an exam under anesthesia. A CT may replace chest x-ray and IVP for assessment of retroperitoneal nodes, hydronephrosis and rectal involvement. PET/CT may be useful to evaluate extent of pelvic disease as well as nodal dissemination.
MRI predictions are based on small studies with few patients. What has been published seems to demonstrate that MRI using sequential volumetric analysis may predict overall response. In patients with a repeat MRI at 30 Gy, those with response predicted local tumor control.
Imaging
FIGO staging does not allow advanced imaging because of concerns about a lack of universal availability. CT can be used as part of the staging to assess hydronephrosis as a surrogate for pelvic sidewall extension, advancing the FIGO stage to IIIB
MRI is more accurate for determining local extent of disease, including parametrial and vaginal involvement with 90% accuracy.
PET/CT is used for nodal staging and distant disease identification at 85% sensitive and 95% specific.
General Management and Treatment
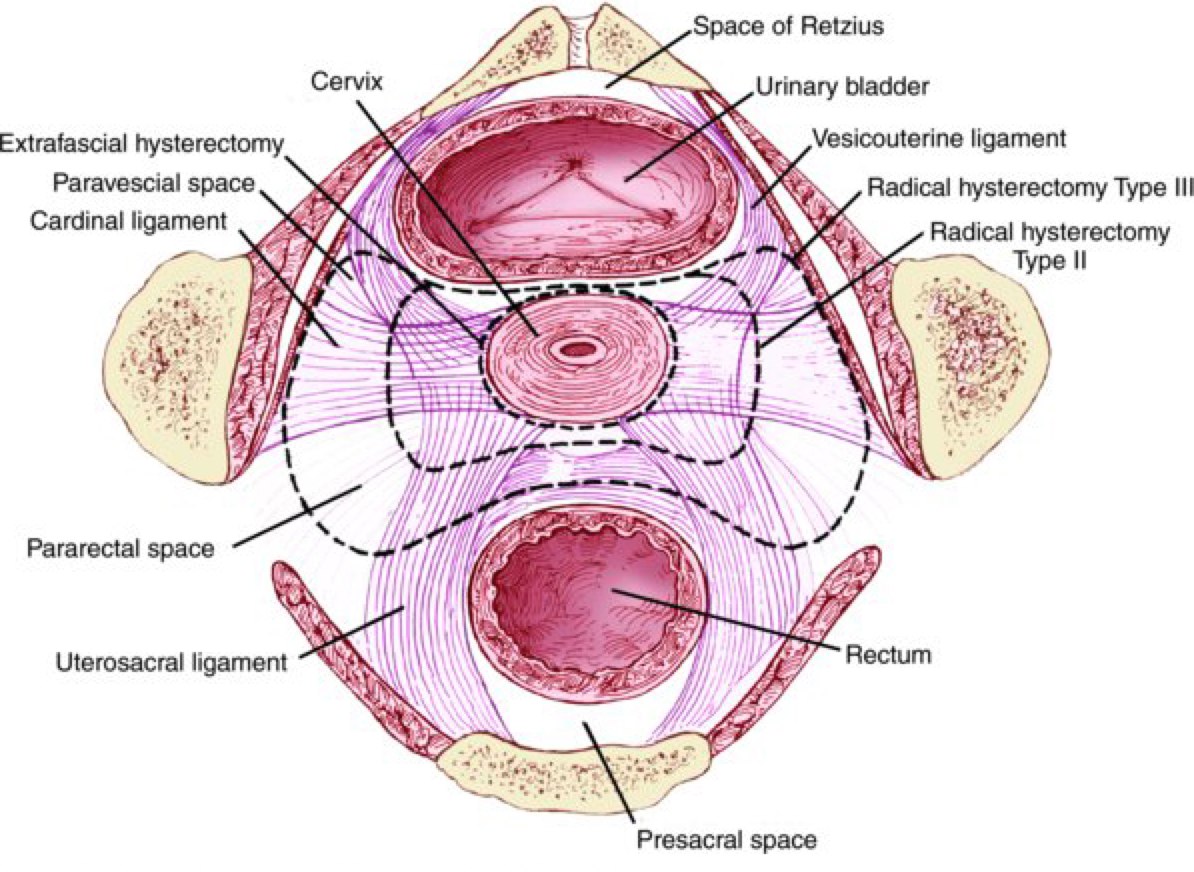
Early cervical cancer can be treated with hysterectomy or in the case of desired future fertility in select cases, treated with trachelectomy alone in highly select situations. The nature and nomenclature of hysterectomies is:
Surgical Techniques and Terminology
- LEEP
Excision using a loop electrocautery excision. This is used in localized disease only.
- Cold Knife Cone
- Sharp dissection of the distal cervix, removing approximately 1/2 of the distal cervix. ECC should be used on the remaining endocervix.
- Radical Trachelectomy
- Removal of cervix. This is used in women who desire future fertility in very localized cervical cancer. Appropriateness for trachelectomy should be confirmed by advanced imaging, including pre-operative PET to ensure the patient is node negative. An MRI can be used to confirm the endocervical canal is free of disease. A permanent cerclage is placed at the upper os region to support a potential intrauterine pregnancy.
- Hysterectomy - Total
- Also known as an extrafacial histerectomy removes the cervix, adjacent tissues and a small portion of the upper vagina, outsed the pubo-cercical fascia. Used most commonly in surgical management of Stage IA1 (microscopic < 3 mm deep, < 7mm wide.)
- Hysterectomy - Modified Radical
- Removal of 1 - 2 cm of vaginal cuff, removal of the cervix, parametrium and all tissue medial to the ureters. Usually pelvic lymph node dissection is included with this procedure. Modified radical hysterectomy is most commonly used with Stage IA2 (up to 5 mm deep, 7 mm spread).
- Hysterectomy -- Radical with Pelvic Lymph Node Dissection
- Wide resection of the parametrial tissues with short pedicles to the pelvic wall. Dissection and mobilization of the ureters, mobilization of the bladder and rectum. Removal of a 2-3 cm vagincal cuff and uteral-sacral ligaments is performed. An extended radical hysterectomy includes dissection of the ureter from the vesico-uterine ligament, sacrifice of the superior vesicle artery and removal of the upper 3/4 of the vagina. The extended radical hysterectomy has significant morbidity and high rates of fistula and is rarely performed.
The 2007 Consensus conference changed the nomenclature for hysterectomies based on the extent of pelvic sidewall resection (lateral extent of surgery). The consensus definitions are:
- Type A: Extrafascial hysterectomy
- Minimal resection of the paracervix medial to the ureters and minimal resection of the vagina, less than 1 cm.
- Type B: Modified Radical hysterectomy
- Partial resection of the vesicouterine and uterosacral ligaments, unroofing of the ureter, transection of parametrial tissue at the ureter, and removal of at least 1 cm of vaginal cuff. (B1 = without removal of paracervical nodes, B2 with removal of lateral paracervical nodes).
- Type C: Classical Radical Hysterectomy
- Removal of the entire uterosacral and vesico-uterine ligaments, 1.5-2 cm of upper vaginal with paracolpos excised.
- Type D: Complete Radical Hysterectomy to the pelvic sidewall.
- Includes sacrifice of the hypogastric (internal iliac) vessels, exposes the sciatic nerve. Type D2 also removes fascia and lateral muscles, also known as a laterally extended endopelvic resection.
There are several known risk factors (high risk) for cervical cancer. These risk factors include:
-
Post hysterectomy positive margins
- good margins are ≥ 1 cm (recurrence risk 11%)
- close margins are between 0 and 1 cm (recurrence risk 20%)
- positive margins (recurrence risk 38%)
- parametrial involvement
- positive pelvic lymph nodes
- positive para-aortic lymph nodes
There are intermediate risk features.
- depth of cervical stromal invasion
- lymphovascular space invasion
- tumor size
Treatment Selection Factors: What should we do?
Landoni Stage IB/IIA Surgery ± radiation v. radiation alone
Landoni did a study of 343 patients with Stage FIGO IB/IIA randomized between surgical management with post-operative radiation only for high risk factors (Positive lymphatics, deep stromal invasion, positive margins). The other arm of the trial was external beam radiation to 50.4 Gy plus brachytherapy.
Over half of the radical hysterectomy arm received PORT.
The resuls were comparable disease free sruvival (DFS5) at 80% (surgical) v. 82% (radiation alone) (NS), and OS5 was 87% (surgical) v. 90% (radiation alone) (NS). Grade 2-3 toxicity was double that of radiation alone at 28% v. 12%.
Outcomes were the same, but toxicity was worse in the surgical arm.
GOG 92: Sedlis/Rotman FIGO IB Intermediate Risk Surgery (Radical Hysterectomy)± Radiation
This study looked at 277 patients treated with radical hysterectomy, and PLND with intermediate risk factors: LVSI, deep stromal invasion, LVSI with middle third invasion and > 2 cm, LVI and superficial 1/3 invasion > 5 cm, or no LVI, deep or middle 1/3 stromal invasion and > 4 cm.
These patients were randomized to Radiation, 50.4 Gy or observation alone.
The Sedlis/Rotman GOG 92 demonstrated improved progression free survival with the addition of post-operative radiation therapy. This study has been used to discount the role of radiation by suggesting it showed no survival benefit. The study was not powered to show a survival benefit. It was powered to show that it prevents local disease recurrence. Radiation significantly increased profression free survival (p=0.009). Beyond 6 years, only for disease related deaths occurred. The convergence of survival curves at 12 years is due to other causes.
Conclusion: Radiation significantly improved progression free survival.
GOG 109: Peters: Surgery → Post-Op Radiation ± Chemotherapy for high risk factors
This trial looks at patients treated with radical hysterectomy plus pelvic lymph node dissection followed by post-operative radiation ± chemotherapy. High risk patients were defined as those with any of these:
- Positive Nodes
- Positive Margins
- Parametrial disease
Radiation was delivered at 1.7 Gy/day to 49.3 Gy. In the concurrent chemotherapy arm, CDDP (70 mg/m2) with 5FU (1000 mg/m2) every 3 weeks.
Chemotherapy + Radiation improves overall survival and as a result is now the standard of care.
Summarizing:
- Landoni: Surgery often required PORT, but RT did not require surgery and the surgical arm was more toxic.
- GOG 92/Sedlis-Rotman: Surgery → Radiation + chemotherapy improved PFS.
- GOG 109/Peters: Post-op Radiation + chemotherapy improves overall survival and is now the standard of care.
- Women with large tumors, deep stromal invasion and/or LVSI should be considered for adjuvant radiation
- Women with positive margins, positive nodes or positve parametrium should be offered radiation with chemotherapy (concurrent)
GOG 263 will assess Radiotherapy ± chemotherapy (carbo/taxol) in intermediate risk women.
Treatment Selection: Locally Advanced Disease
RTOG 9001/Morris/Eifel: Stage IB-IIA > 5 cm, IIB/IVA or Pelvic Node Positive Disease with para-aortic nodes
Randomized to 45 Gy at 1.8 Gy/fraction + Brachytherapy to a median dose of 87 Gy to point A, or whole pelvic radiation with CDDP (75 mg/m2) + 5FU bolus every 3 weeks x 3 cycles + brachytherapy.
The study demonstrated that radiotherapy to the whole pelvis only plus a brachytherapy boost with chemotherapy improved overall survival when compared with extended field (EFRT) radiation to the para-aortic nodes. The acute toxicity was worse with concurrent chemotherapy/radiotherapy, with 11% grade 4-5, v 1%, but the majority was due to hematotoxicity. Late toxicity was 14% in both arms.
GOG 165 (Lanciano) compared weekly CDDP (40 mg/m2) v. continuous infusion 5FU
This trial was stopped ealry due to worse outcome in 5FU arm.
Conclusion: 5FU is inferior to cisplatin.
GOG 120/Rose: weekly CDDP (40 mg/m2) v. + 5FU + hydroxyurea
CDDP alone was better and 5FU only added toxicity.
GOG 85/Whitney: hydroxyurea v. CDDP + 5FU x 2 cycles
CDDPis better.
NCIC/Pearcey: weekly CDDP (40 mg/m2)
Inferior trial.
Conclusions
- CDDP (40 mg/m2) is the most toleratble regime for concurrent Radiotherapy and Chemotherapy treatment.
- There is no evidence that 5FU improves outcomes and there is evidence that it increases toxicity.
- Hydroxyurea is inferior to CPPD based treatment.
Adjuvant Chemotherapy after Concurrent Chemotherapy and Radiation
An Argentine trial randomized 515 patients to weekly CDDP + radiation therapy or gemcitabine+CDDP with RT → Gem+CDDP x 2 cycles after concurrent radiation/chemotherapy. There was improved disease free survival and overall survival with the addition of adjuvant Gem+CDDP, but there was also increased toxicity. It is not clear whether this is a chemotherapy trial: was the benefit from the addition of gemcitabine or from the adjuvant chemotherapy?
Australian Outback Trial: Concurrent radiation + CDDP + Brachy ± Adjuvant chemotherapy (Carbo/Taxol x 4 cycles)
The results are not yet in.
Treatment Duration and Impact on Outcomes
Time is of the essence. In patients treated with radiation therapy, the overall elapsed treatment time is critical. Unplanned interruptions or delays in treatment should be avoided. Integration with intercavitary implant and external beam radiation is important. Overall treatment time was the most highly significant predictor of local-regional control. Treatment time overall should be less than 7 weeks (35 days) when radiation alone is used. Fyles reported a 1% loss of control in patients treated with radiation alone when treatment time extended beyond 30 days. Perez reported that completing all intracavitary brachytherapy within 4.5 weeks (23 days) of initiation of radiation therapy improved lower pelvic failure rates from 18% to 8.8%.
Shaverdian et al reported that treatment duration was not correlated with in-field failure, disease free survival or overall survival when treated with chemotherapy and radiation therapy (CRT).
Summary for Early Cervical Cancer
| Stage | Actions | |
|---|---|---|
| IA1 (Cervix only ≤ 3mm deep x 7 mm) | Hysterectomy (or CKC if future fertility desired) | |
| IA2 (Cervix only 3 mm - 5mm invasion) | Hysterectomy and PLND. If LVSI, then hyst is required with nodal staging | |
| Radiation reserved for inoperable patients | ||
| IB1 (≤ 4 cm) | Radical Hysterectomy Type B/C (to ureters or PSW)
Alternatively, Primary Radiation ± CDDP (reasonable to add, but limited data) | |
| IB2 | Primary Radiation with Chemotherapy preferred | |
High Risk/Locally Advanced Cervical Cancer Summary
| Stage | Actions |
|---|---|
| II/III | Primary CDDP/Radiation. Rad hysterectomy has higher toxicity than radiation with similar outcomes. |
Women with large tumors, deep invasion and/or LVSI hould be considered for adjuvant radiation. If margins are positive, nodes or parametrium are positive, then add CDDP chemotherapy.
Radiation Therapy Treatment Planning And Techniques
Drawing on RTOG standard arms, RTOG 0724 for post-radical hysterectomy early cervical cancer, and the Outback Trial (ANZGOG 0902) as a reference, Generally accepted regimens are 45 - 50.4 Gy for external beam, followed by an intercavitary implant using either ring and tandem or tandem and ovoids to boost the cervix.
Indications for Post-operative Radiation Therapy
The usual indications for post-operative radiation therapy alone include:
- lymphovascular space invasion
- deep stromal invastion (≥ 1/3 depth)
- tumor > 4 cm (FIGO IB2)
The usual indications for post-operative concurrent chemo-radiation therapy include:
- Positive margins
- Positive lymph nodes
- Parametrial extension or greater. (FIGO IIB/IIIB)
External Beam Radiation Pelvis and extended fields
Radiation Doses and Timing
External beam radiation therapy is usually initiated to shrink the tumor to < 4 cm. Once the tumor is less than 4 cm, then brachtherapy can be used to boost the cervix. This is generally after about 20 Gy. The external beam doses are generally in the range of 45 - 50.4 Gy at 1.8 Gy/fraction. Some modern studies use 50.4 Gy to the pelvic nodes and parametrium using IMRT techniques (Small).
Traditional radiation fields include the whole pelvis to 45 Gy, with a boost to the sidewall to 50-54 Gy. If there is residual or bulky parametrial tumor, this can be further boosted to 60 Gy. If there is indication to treat the PALN, then 45 Gy is used, with a boost to 60 Gy for positive nodes. Generally IMRT is now used to treat extended fields.
Treatment Setup and Simulation
Most patients are treated supine with VacLock immobilization. Prior to simulation, fiducial markers can be placed in the cervix under sedation and local anesthesia. If there is vaginal extension of disease, additional fiducials may be placed in the vagina at the distal extent of disease. The inferior border should be between 3 and 4 cm below these markers on 3d-CRT treatment plans.
At the time of simulation, a means of marking the vagina is useful. A large rubber insert is commercially available, but this distends and displaces the vagina and should not be used. A barium soaked applicator which conforms to the vagina or a raytex sponge with a dilute contrast agent can be inserted. Rectal contrast should be used. Dilute contrast to help identify the bowel is helpful. IV contrast may be useful, if available. Imaging should be done initially with bladder reasonable full. It is helpful to have the patient drink 32 oz of water 1 hour prior to simulation and at the same time prior to treatment each day. A second post void scan should be taken and the post void images should be fused to determine the extent of displacement with full and empty bladder. Full bladder treatments are useful to displace the bowel from the treated fields.
Generally for 3d-CRT treatments, a 4 field technique is used. The upper border for node negative patients is L4/L5. If the pelvic nodes are positive or common iliacs are positive, contour the pelvic nodes and the common iliacs. The upper border is then set at L3/L4. If the para-aortic nodes are positive, use extended field radiation with the upper border set to T12/L1. In this situation, a PET/CT may be very helpful to determine the upper extent of positive nodes. These nodes should be contoured for potential boost radiation. Kidneys and other organs at risk should be identified and shielded. The lateral borders should be 1 cm lateral to the
EBRT
The Outback Trial (Australia) does not permit IMRT. It's goal is to ensure that the trial is truly multi-national and the techniques used can be successfully used in developing nations. The trial uses doses of EBRT to 45 Gy - 50.4 Gy whole pelvis. If the common iliacs are involved, then the field is extended superiorly. A 4 field box treatment is required using APPA/R/L Lateral fields. The primary reason given for the restriction on IMRT/RapidArc is the limited QA available for the study.
Doses to the whole pelvis is 1.8 Gy to 45 - 50.4 Gy. Where EFRT is required, the dose is 45 Gy to the extended field.
If brachytherapy is not available, shrinking field techniques are permitted for boost fields. The goal is to treat the gross tumor volume with a margin to a minimum dose of 65 Gy. An attempt to exclude small bowel after 50.4 Gy should be made. Parametrial/pelvic nodes may be boosted if they are PET or CT positive. CT positive is defined as 1.5 cm on short axis or histologically confirmed positive by biopsy/sampling.
Parametrial and/or nodal boost is up to 10 Gy with the total pelvic sidewall dose not to exceed 65 Gy at the mid-plane. For parametrial boost, the field should include only the true pelvis. The superior border of the boost field is 1.0 cm above the inferior aspect of the sacro-iliac joint. If nodes are positive, add a boost to the positive node plus 2 cm margin around the gross nodal disease and delineated on CT, PET or MRI. Nodal boost can be delivered via 3d CRT. Timing of boost is after EBRT is complete and between brachytherapy treatments. Overall treatment time should be kept to 7 weeks or less, if possible.
GTV is defined as the gross tumor volume which includes primary cervical cancer, and gross extensions and any grossly involved lymph nodes. The CTV includes the GTV, parametria, uterus, upper half of the vagina, internal, external and distal common iliac nodes and the utero-sacral ligaments.
Fields
The APPA fields should cover at the superior aspect, the L4/L5 interface or L5/S1 space. The lateral borders are 2 cm lateral to the true pelvis and the inferior borders are at the inferior margin of the obturator foramenae, unless lower is required for vaginal disease extension. This border should be set to 3 cm inferior to the inferior most vaginal disease extent. The femoral heads should be protected.
 |  |
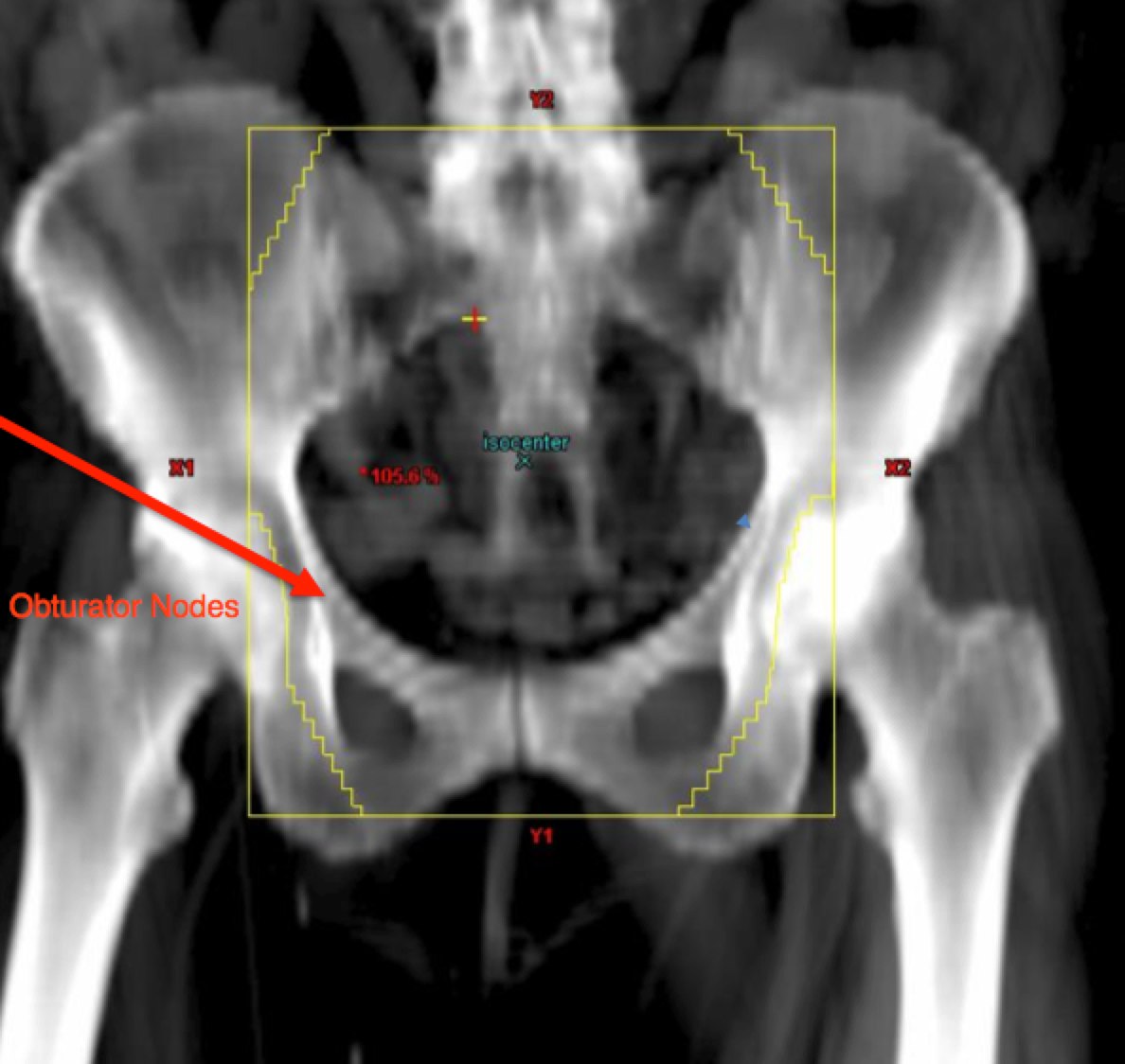 | 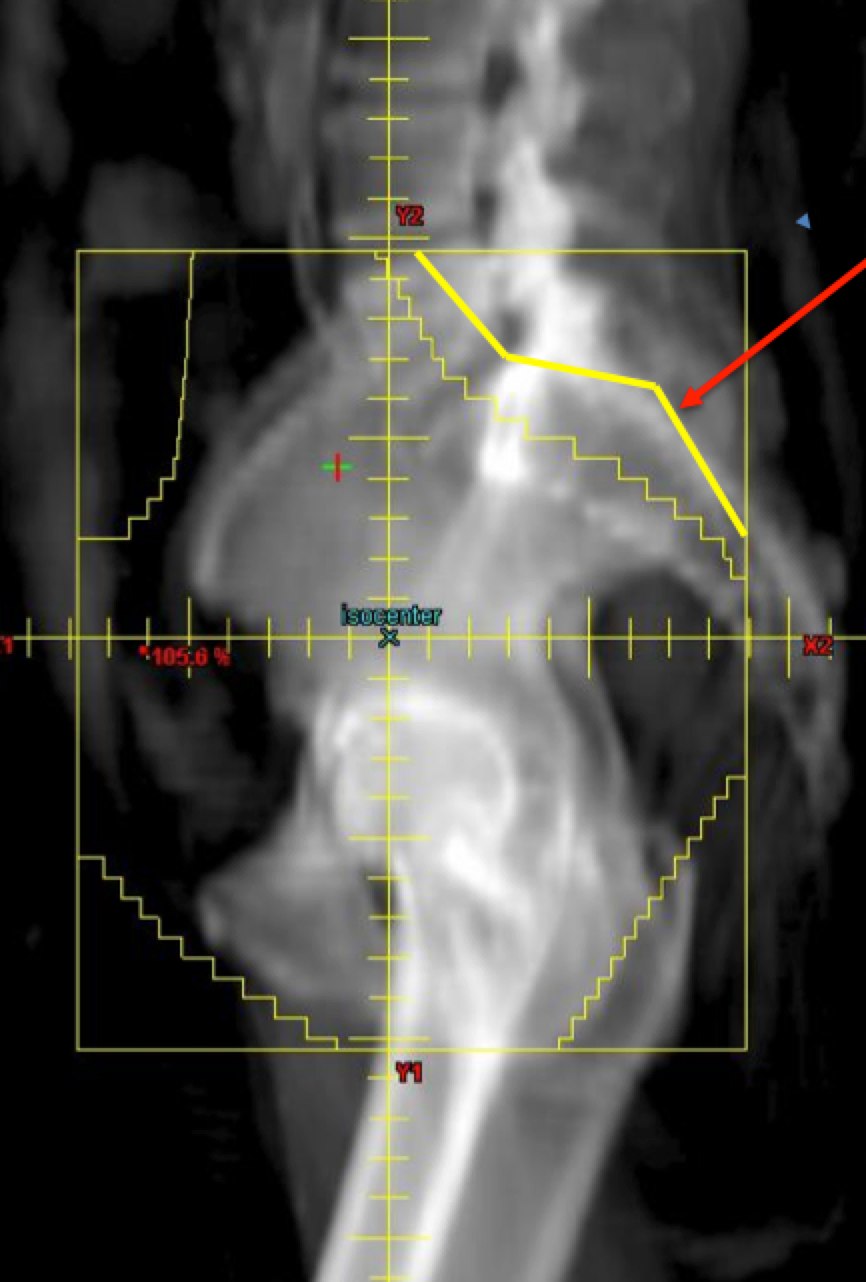 |
Lateral fields should have the same upper/lower borders as the AP/PA fields. The anterior border should be 1 cm anterior to the most anterior point of the pubic symphasis. The posterior border should intersect S2-S3 or 3 cm posterior to the uterus or gross disease. Blocking should be designed such that there is at least a 2 cm margin. The outer half of the sacrum may be blocked to protect the sacra plexus on lateral fields.
EFRT APPA Fields should encompass the highest involved node with a margin of 3 cm or one vertebral body above the node. Lateral margins above L4 should be at least 6 cm wide or 2 cm lateral to the lateral border of the involved node. PROTECT THE KIDNEYS!
EFRT Lateral Fields should be 3 cm anterior to the anterior aspect of the vertebral body or 2 cm anterior to the anterior aspect of any involved node. The posterior aspect should be 5 mm - 7 mm anterior to the cord, if the involved nodes can be safely covered.
The Para-aortic Field
When do we treat it?
For the following patients:
- Patients with imaging confirmed pelvic or para-aortic lymphatics (CT or PET)
- Superior border is extended to L1-L2 interface
- Cover the tips of the lumbar transverse processes which will be about ˜ 5 cm wide.
- Cover at least 2 cm from the anterior aspect of the vertebral body
- Cover at least 1/2 of the vertebral body
- Contour and protect the kidneys!
- If the common iliacs are positive, cover the extended field.
Most of the positive nodes are in the LEFT para-aortic region followed by the aorto-caval region. Few are located to the right of the IVC (inferior vena cava). Special attention should be paid to hte space on the LEFT LATERAL aspect of the aorta.
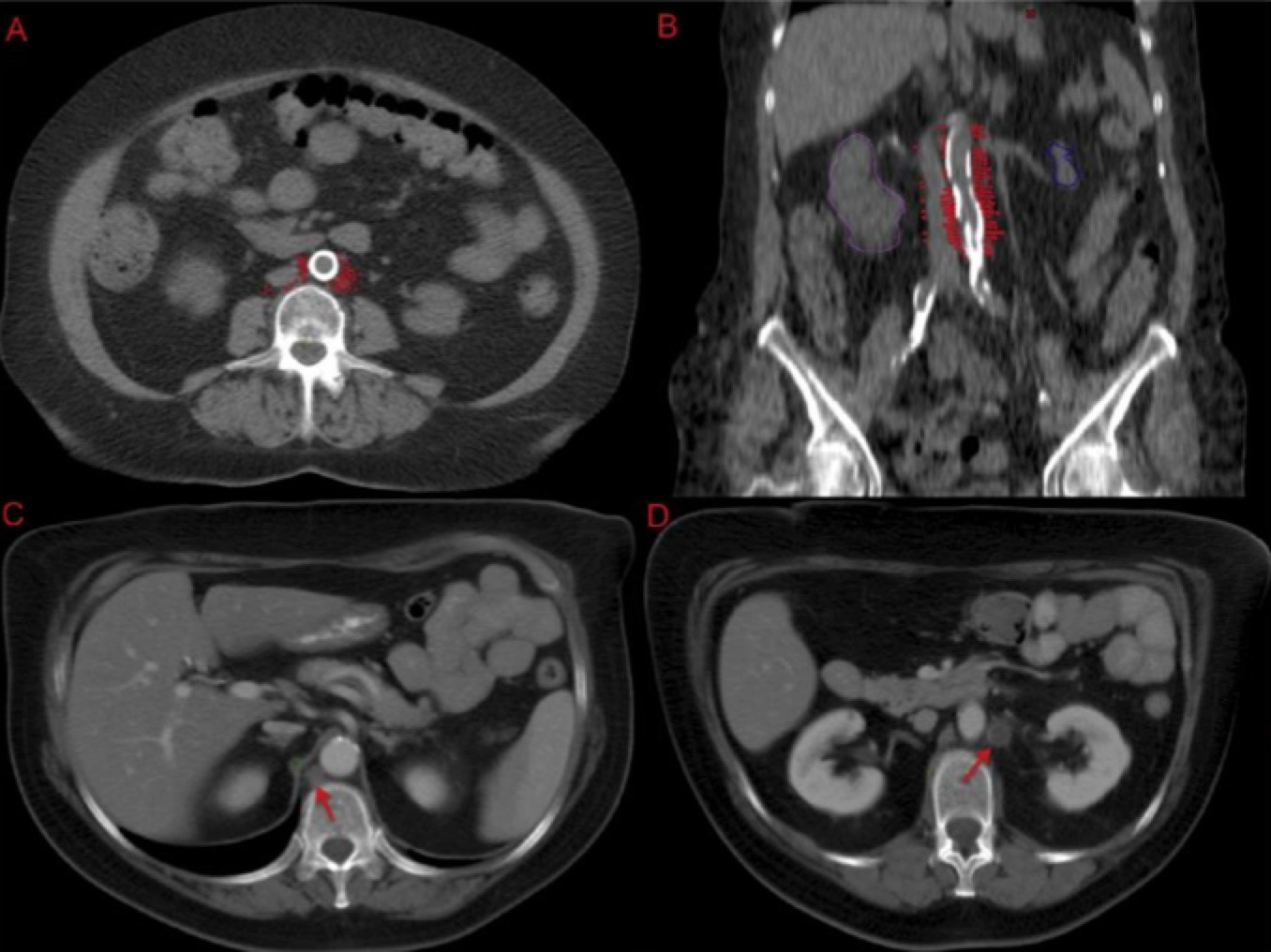 |
Brachytherapy Boost Vaginal Cuff Boost for POST-OPERATIVE Cases
If a vaginal cuff boost is given in the post-operative Cervix, it should follow external beam radiation and be initiated within 7 days of completion of pelvic radiation. If HDR is used, more than one dose may be given in a week and external beam and brachytherapy boost should not be given the same day. Treat only the vaginal cuff and do not include more than the upper 2/3 of the vagina. Cylinders or colpostats may be used. The following dose schedule is recommended:
Post Op doses are generally
- HDR: 2 - 3 applications at 6 Gy per fraction to the vaginal surface for a total of 12 - 18 Gy.
- if the pelvic dose is 45 Gy treat with 3 fractions to 18 Gy (combined dose 63 Gy)
- if the pelvic dose is 50.4 Gy treat with 2 fractions to 12 Gy (combined dose 62.4 Gy)
- LDR: low dose brachytherapy to 20 - 25 Gy to the vaginal surface at a dose rate of 0.8 - 1.2 Gy/hr with final dose determined by:
- if the pelvic dose is 45 Gy treat with LDR to 25 Gy (combined dose 70 Gy)
- if the pelvic dose is 50.4 Gy treat with LDR to 20 Gy (combined dose 70.4 Gy)
Brachytherapy Boost for NON-surgical Cases
For non-surgical (Radiation alone/chemotherapy+Radiation therapy) treatment, the brachytherapy consists of ring and tandem or tandem and ovoid treatments. The goal is to bring the Point "A" dose to the following levels:
| Stage | Dose to Point A | EBRT Dose to Pelvic Sidewall |
|---|---|---|
| Stage IA | 65 Gy - 70 Gy | 45 Gy |
| Stage IB1-IIB | 75 Gy - 85 Gy | 45 Gy - 50.4 Gy (reduce superior border to L5/S1 at 45 Gy) |
| Stage III-IVA | 85 - 90 Gy | 50.4 Gy - 60 Gy (reduce superior border to bottom of sacro-iliac joints at 50.4 Gy) Treat to 60 Gy only if persistent parametrial tumor is seen. |
The Pelvic Sidewall Boost
A pelvic sidewall boost may be added to improve lateral coverage for bulky IIB/IIIB disease. This should be delivered at 1.8 Gy - 2 Gy for 2 to 4 fractions (45 Gy → 50.4 Gy ). Be very aware of combined doses to the sigmoid/rectum and bowel. Consider omitting if there has been a good response to concurrent radiation and chemotherapy. Interstitial implant needles may reduce the need for a PSW boost. Use a midline block, if treating conventionally and drop the upper border to the top of the lower pelvis.
IMRT Techniques (RTOG 0724 Post-OP)
Overview
Definitive techniques were studied by the RTOG. For post-operative cases, the RTOG has combined both uterine and cervical cancers for the purposes of determining appropriate target volumes, doses, and organs at risk. IMRT dose specifications have include parameters for dose inhomogeneity within the target volume, within the avoidance structures, and safe dose-volumes for organs at risk.
The RTOG and others have developed reference anatomical atlases to describe pertinent structures. Often, perhaps too often, these atlases focus on the clinical target volumes, with lesser emphasis on normal anatomy which helps define the field and identify the organs at risk.
Definitive IMRT Radiation Therapy Dose Specifications — RTOG 0724/Post-Operative IMRT
Doses are generally given to either 45 Gy or 50.4 Gy at 1.8 Gy/fraction. Boost doses to grossly positive residual disease have also been advocated. Fields typically follow traditional superior borders, except where there is positive common iliac nodes and/or para-aortic nodes. The GOG has demonstrated an advantage to extended field radiation therapy to the level of T12/L1 or T11/T12 when nodes are involved. Cervical cancer with positive PALN (para-aortic lymph nodes) has a dismal prognosis compared to earlier stages with DFS5 95% for node negative, 75% for lower pelvic nodes only and 33% for positive para-aortic nodes. When the supraclavicular (Virchow's) nodes are involved, DFS5 drops to around 10%.
Dose Specifications
Radiation treatments should be started within 70 days after surgery, or before post-op week 10. Sooner is preferred. Treatment breaks should be avoided, if at all possible. Treatment frequency should be 5 fractions per week, once daily. Dose is 45 Gy at 1.8 Gy/fraction in 25 fractions or 50.4 Gy at 1.8 Gy in 28 fractions. The 97% isodose volume should cover the vaginal and nodal PTV. The PTV is constructed by adding a 7 mm margin around the ITV, which accounts for organ motion. Homogeneity should be maintained to less than 110% single pixel (0.03 cm3 hot spots.) Cold spots should not be lower than 93% isodose volume to a single pixel. Contiguous volumes outside of the target volume must not be > 110% hot.
The following targets should be defined:
- vagina
- Parametria
- Pelvic Lymph nodes
- If EFRT is indicated then the PALN
Organs at Risk
For cervical cancers treated to 45 - 50.4 Gy, The RTOG 0724 Trial recommends the following dose limits.
- Bowel:
- No more than 30% of the volume > 40 Gy
- no volume > 0.03 cm3 higher than 110% of prescription dose (49.5 Gy - 55.44 Gy)
- Rectum:
- No more than 60% of the volume > 45 Gy and
- no volume > 0.03 cm3 higher than 110% of prescription dose (49.5 Gy - 55.44 Gy)
- Bladder: no more than 35% of the volume > 45 Gy and no volume > 0.03 cm3 higher than 110% of prescription dose (49.5 Gy - 55.44 Gy)
- Kidneys 2/3 of each kidney ≤ 18 Gy
- Spinal Cord ≤ 45G to any volume ≥ 0.03 cm3
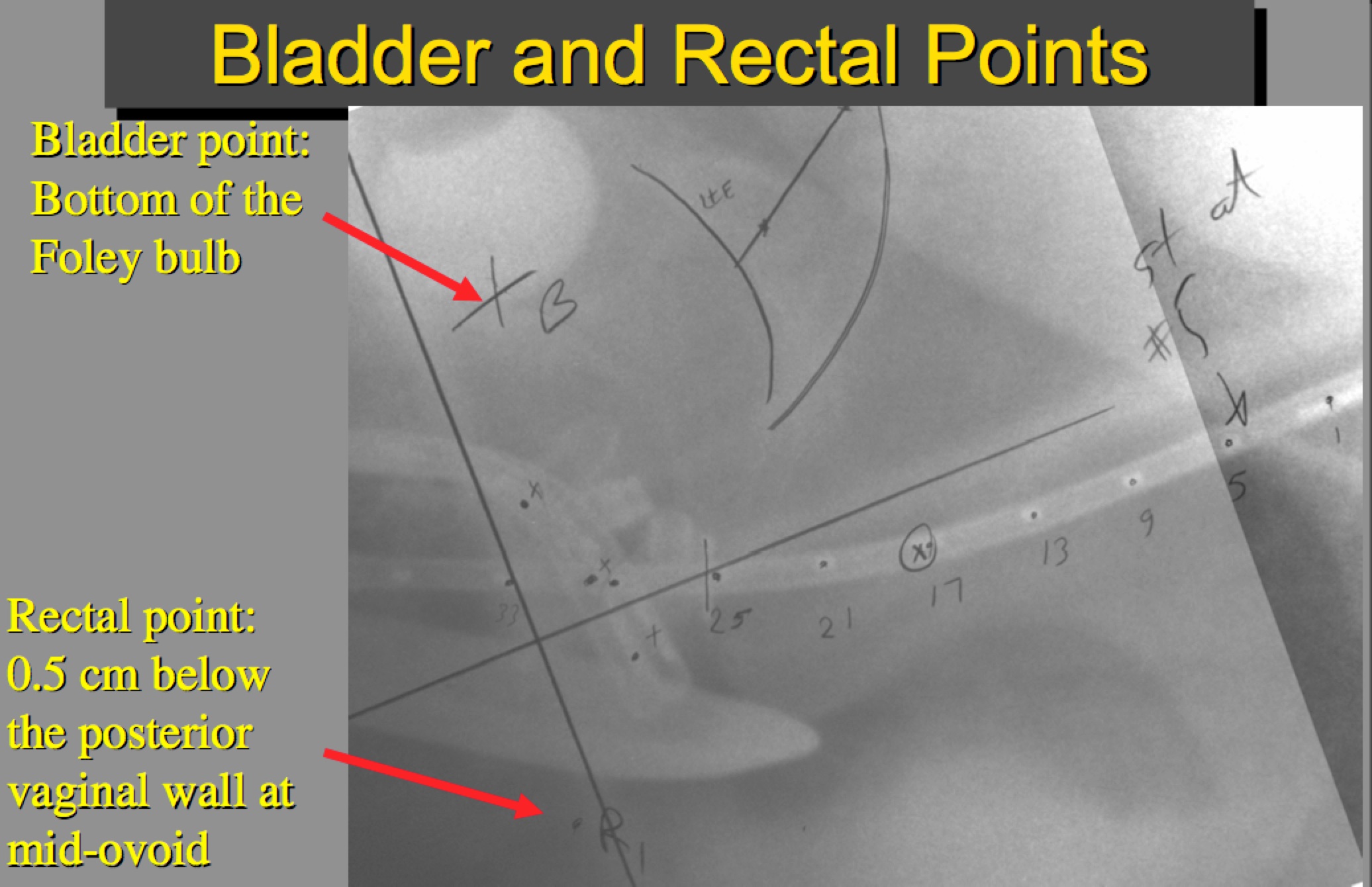
ICRU Bladder dose point is constrained to ≤ 75 Gy. The Bladder point is defined as the bottom of the foley bulb.
ICRU Bladder point is constrained to ≤ 70 Gy.
External Beam Radiotherapy Based on RTOG 0724 Trial
RTOG 0724 is based on earlier GOG trials (Peters, Rose, Morris). These trials demonstrated an advantage to adding platinum based chemotherapy to radiation therapy in cervical cancer. They reported a decrease in recurrence rates from 31% (radiation alone) to 13% with concurrent chemotherapy. RTOG 0724 tests concurrent platinum and radiation → carbo/taxol chemotherapy v. observation. It stratifies by two dose scales, 45 Gy and 50.4 Gy, IMRT v. Conventional 3D-CRT and also by intention to treat with or without brachytherapy.
IMRT Techniques
ESTRO recommends MRI Based Treatment Planning.
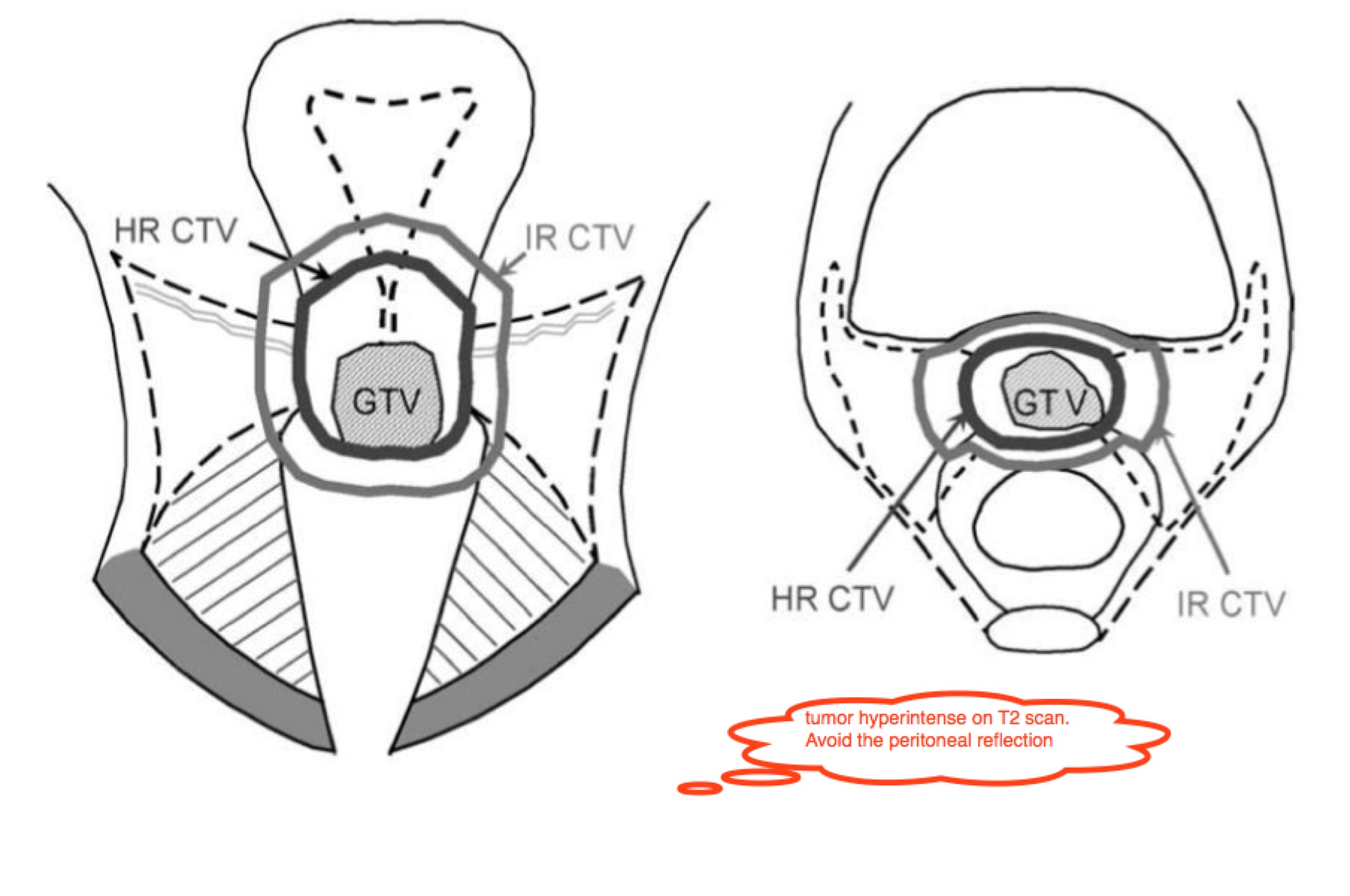
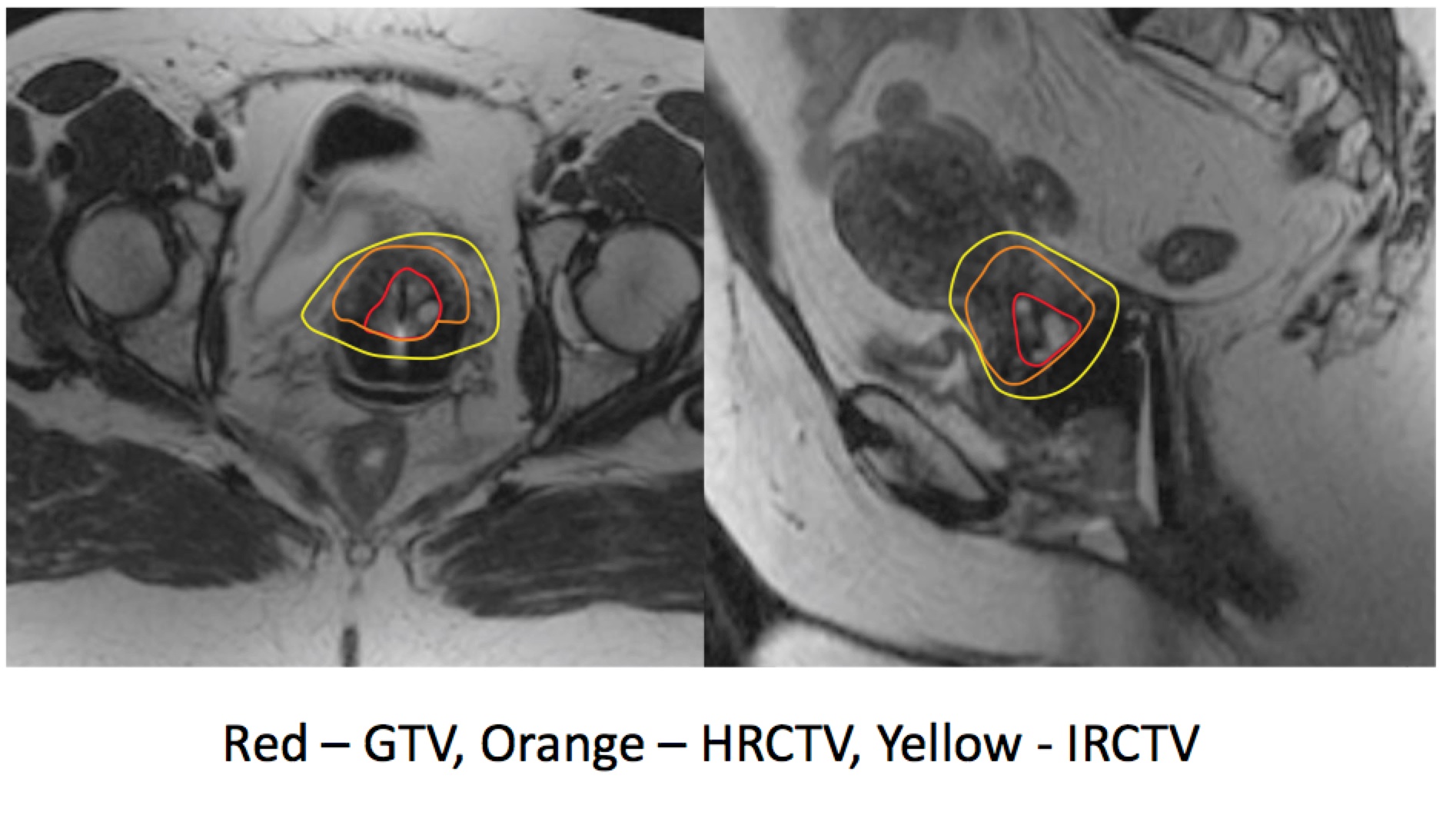
Brachytherapy Dose Points
Outcomes, Patterns of Failure, Prognostic Indicators
Nodal Status
Node status is an important determinant of prognosis. Cause specific survival is a function of stage and node status. Grigsby has reported in detail on the prognositic indicators of Stage and node positivity. (Grigsby JCO 2010; 28:12 pp 2108-2113.) This report examined about 560 patients and analyzed by nodal status (N0, pelvic nodes only, para-aortic nodes), as determined by PET/CT for outcomes between 2000 and 2009.
Grigsby offers the conclusion that the most significant predictor of progression free survival was the presence of positive para-aortic lymph nodes on PET/CT imaging. His key findings:
- The frequency of PET positive lympathics is a function of stage
- PET nodal staging correlated well with reported surgical nodal staging studies
- Extent of PET identified nodal involvement corresponds to distinct prognostic oucomes, suggesting that PET idenfied nodal disease influences
- Recurrence free survival and disease specific survival is dependent on the most distant nodal metastases
- This RFS/DSS pattern was independent of stage.
- 47% of patients in this study were node positive (PET) at diagnosis:
|
||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||
Age, Race and Socio-economic Status
Age is controversial as a prognostic indicator. European studies showed improved outcome for younger patients. Others have not. Comorbid conditions precluding brachytherapy may explain some of this difference. Race/SES has been studied in N. America by Arno Mundt. Factors associated include lower Hb, lower income, less frequent intracavitary implant brachytherapy. Multivariate analysis did not show that race was a factor, after controlling other differences.
General Medical Factors
Anemia and tumor hypoxia influence prognosis. Transfusion benefits are transient, and if used, transfusions should be given in close proximity to radiotherapy doses. Pre-treatment anemia has been shown to impact 3 year relapse rates.
Tumor factors
HPV: there is a higher risk of node involvement with HPV18.
Tumor volume: There is a close relation between stromal invasion, tumor size and incidence of parametrial and pelvic nodal metastases.
Surgical Factors
Martin status, positive parametrial spread and positive lymph nodes all are adverse predictors.
Treatment duration
In patients treated with radiation therapy, the overall treatment time is a factor in local-regional control. Timely integration of brachtherapy with external beam radiation is important. Overall treatment time should be kept to less than 30 days. Integration of brachytherapy in the final weeks of external beam should be considered.
Survival Statistics
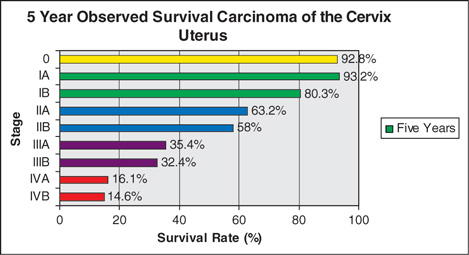
Stage by stage survival gains have been incremental over the decades, but the incidence of advanced stage disease is changing due to early and effective screening. Most cervical cancers are detected in the developed world as CIS. Survival for cervical cancers have improved by 18% over the past half century. Cure rates for early stage disease exceeds 90%.