Non-Invasive Breast Cancers — DCIS
DCIS Cases
DCIS
DCIS is a disease that is confined to the ducts of the breast. These cells have not demonstrated an ability to cross the basement membrane of the ducts and lack the ability to invade and involve the surrounding breast stroma. Axillary node involvement is rare, ranging from 0% to 5%. When axillary nodes are involved it is usually because there is a focus of undiagnosed microinvasion within the DCIS.
Epidemiology and Risks
Risk factors are generally similar to those of invasive breast cancers, which include:
- Family history
- Reproductive history (delayed age of first pregnancy, mulliparity)
- History of benign biopsies
- Some think dietary factors such as increased ethanol consumption, however, there are many potential compounding factors
Increased mammographic detection at earlier stages has resulted in a stage and demographic shift at detection. From 1983 to 2004, the annual incidence rate shifted from 4800 cases to > 50,000. 290,000 new breast cancers are now detected annually with approximately 58,000 non-invasive. Of these 85% will be DCIS. Of the DCIS subset, most will not be palpable in women who recieve routine screening. The rate of screening dectected DCIS increases with age, despite the fact it accounts for a smaller percentage of breast cancers overall.
- Rate of DCIS in women 40 - 49 years: 0.56 / 1000 mammograms
- Rate of DCIS in women 70 - 84 years: 1.07 / 1000 mammograms
DCIS is generally discovered on screening mammogram. Prior to widespread screening mammography DCIS presented as a palpable lump or nipple discharge. Today, with the advent of mammography, nipple discharge is not commonly a presenting symptom and screening mammograms are capable of detecting less than palplable lumps. With the increased sensitivity of mammographic screening, breast cancer free survival rates are now approaching 100%.
Pathology and Natural History
DCIS is defined by the presence of malignant epithelial cells within the well defined ductal space of the breast. They are bound by and do not cross the basement membrane of the ducts without myeloid invasion. DCIS has several architectural subtypes:
- solid
- comedo
- micropapillary
- papillary
- cribriform
DCIS is further characterized by grade (high, intermediate and low), and whether or not there is the presence or absences of necrosis.
Due to the heterogeneous nature of DCIS several schema have been presented as possible means to address the need for and nature of treatments offered. There is not clear data available on prognostic factors. Retrospective data has shown that there is an increased propensity for local recurrence after breast conserving surgery in:
- younger women
- comedo histologies
- high grade disease
- close or positive surgical margins
Despite this there is a lack of comprehensive data on these prognostic indicators. Several prognostic tools have been published, such as the "Van Nuys" criteria, but to date, these have not proven beneficial in selecting (or deselecting) patients for treatment. Given this situation, it is not clear how to incorporate the above factors into the treatment decision process. It also appears clear that older data on clinically detectable DCIS (palpable lumps, nipple discharge) are not directly applicable to post-mammography detected disease. Clinically symptomatic DCIS may not be applicable to mammographically detected cases. Finally, there is a lack of significant data concerning the natural history of untreated DCIS.
Pertinent Breast Anatomy
Breast anatomy of importance in DCIS is the ductal architecture. DCIS rarely spreads beyond the ducts, but it does however, travel along the ducts. The ducts are a branched pattern in the breast lobules which converge at the nipple.
Clinical Workup and Staging
DCIS is staged Tis. The clinical workup includes imaging studies. 95% of all DCIS is now found on screening mammograms. The mammographic presentation is generally a pattern of abnormal microcalcifications. Asymmetric densities make up about 10%, dominant masses another 8%. Suspicious calcifications are found in several moeieties: amorphous, coarse, pleomorphic and fine linear types. Linear and branching types are often found in higher grade DCIS and necrosis, whereas fine and granualr calcifications are more commonly seen in low grade DCIS.
- Initial mammographic evaluation
- Abnormality identified: magnfication spot and compression views to allow for comprehensive characterization of findings
- Determine the need (based on mammography) for biopsy or further evaluation.
- Mammographic extent of disease tends to underestimate true size ⇒ MRI may be useful in high grade DCIS with special techniques
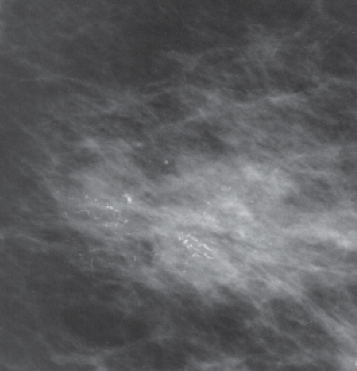 | Linear microcalcifications suggestive of high grade DCIS |
 | Clustered microcalcifications suggestive of low grade DCIS |
Treatment Recommendations
There is a continuum of DCIS disease characteristics which confound decision making, as well as a more recent evolution in the discovery of DCIS at earlier and smaller disease due to the widespread use of screening mammography. Anti-estrogen therapy (tamoxifen and aromatase inhibitors) have been shown to reduce the risk of ipsilateral as well as contralateral recurrences or new instances of disease in some groups of patients. Anti-estrogen therapies have added to the uncertainty in breast cancer management generally and in making local treatment option determinations.
Local treatment includes mastectomy or alternatively, breast conserving approaches. There are no randomized prospective trials examining breast conservation or mastectomy. It is unlikely that such a trial would accrue in sufficient numbers to provide meaningful survival statistics. A retrospective pathologic review of NSABP-B06 identified a number of cases where there was no evidence of invasive cancer, in the setting of DCIS. The outcome of these cases at 83 months (6.9 years) demonstrated a 43% failure rate in patients treated with lumpectomy alone compared with 7% in patients treated with post-lumpectomy radiation therapy. Likewise, the addition of tamoxifen to lumpectomy has been shown to improve outcomes.
Four prospective randomized trials have been reported comparing outcomes in lumpectomy alone compared with lumpectomy plus radiation therapy, post-operatively. All trials treated the breast to 50 Gy at 2 Gy/fraction without a boost. The trials:
| NSABP B17 | 20 year follow up | Lumpectomy ± Radiation (no tamoxifen) |
|
| EORTC 10853 | 10 year followup | DCIS ≤ 5 cm, LE± RT |
|
| UK/ANZ | 12.7 year followup | DCIS: LE ± Tam LE ± RT LE ± RT+Tam |
|
| SweDCIS | 8 year followup | DCIS LE ± RT (split course/negative margin not required) |
|
| EBCTCG Meta-analysis | 10 year followup | Meta-analysis of above studies |
|
What Is the "Right" Dose?
Recently, with the advent of START-B and the NCIC hypofractionated breast treatment regimens, controversy has arise over the appropriate dose and fractionation schemes for the treatment of DCIS. Most recently, the NCIC published in the IJROBP (Lalani et al, IJROBP 2014;(90)5 pp 1017-1024, doi:http://dx.doi.org/10.1016/j.ijrobp.2014.07.026/) a report on long term outcomes of hypofractionation v. conventionally fractionated radiation therapy for DCIS.
In this study, a review of 1609 cases with pure DCIS (index cases 132,093 1994-2003) were reviewed. The authors conclude based on this population cohort, that at 10 years median followup, hypofractionation was not associated with an increased risk of recurrence compared with conventional radiation.
The ACR on the other hand, in its appropriateness criteria does not support hypofractionated radiation in DCIS. So, what do the data from this study really say?
- The Sunnybrook Study is a population cohort study
- The study compared only DCIS/lumpectomy patients with hypofractionated and conventionally fractionated radiation.
- DCIS was treated with hypofractionated radiation in 40%, despite the lack of randomized clinical trials.
- Findings:
- 10 year local recurrence free survival (HF) 89%
- 10 year LRFS (SF) 85% (p=0.03)
- EBCTCG meta-analysis says that radiation reduced 10 year absolute risk by 15.2%
- EBCTCG meta-analysis 10 year risk reduced from 28.1% to 12.9%, or a LRFS10 of 88.1%
- It appears that the conventional fractionation arm is under-performing on the Sunnybrook study.
- Why?
- A review of the data revealed a difference in treatment: the conventional fractionated arm was far less likely to receive a boost.
- With AF regimen,most (54.2% received a boost (usually 10 Gy/5 fractions)
- With SF regimen (50 Gy @ 2 Gy/fx) only 14.7% received a boost.
- Tamoxifen was not accounted for in this study.
- In the entire cohort, 30.4% received a boost.
So, it appears that a boost may be valuable, and may explain the underperforming arm on the Sunnybrook study. The Sunnybrook group reports the following predictors of local recurrence:
- age < 45 (HR 2.6)
- presence of high grade DCIS (HR 3.0)
- intermediate grade DCIS (HR 2.6)
- conventional radiation scheme (v. hypofractionation) (HR 0.8 95% CI). No mention of the role of boost was made.
The Sunnybrook study did not mention the role of the boost but does note the Hathout study at 4.4 years median follow up, scheme (42.56 Gy in 16 fractions, with a 10 Gy boost in 5 fractions) had local control rates of 97% at 4 years.