The next step in the diagnostic workup is to obtain a stereotactic biopsy or excisional biopsy which demonstrated DCIS, high nuclear grade, ER positive, multiple foci, with margins of < 1 mm in lateral and medial aspects.
There are several options available including:
The ACR has assigned appropriateness criteria scales in three groups 1-3 (usually not appropriate), 4-6 (possibly appropriate), 7-9 (usually appropriate). The ACR criteria suggest as a next step as appropriate Mastectomy without SNB (7), Mastectomy with SNB (8), Re-excision without RT or proceeding to adjuvant RT is not recommended and felt to be usually not appropriate. The EORTC and NSABP B17 studies both identified local excision without radiation or positive margins as a significant risk factor in recurrence. The EBCTCG meta-analysis at 10 years demonstrated an absolute risk reduction of 15.2% and a reduction of risk from 28% to 12.9% with the addition of radiation therapy to clear margins in BCS.
This patient went on to have a re-excision to clear margins with no residual disease in the re-excision specimens.
Radiation therapy is indicated in breast conserving surgery. If she had proceded to a mastectomy, the treatment would be complete, whether or not axillary lymph nodes were sampled. Although high grade DCIS occasionally harbors occult invasive cancer and periodically we do find Stage Tis/TXpN1 disease, this situation is rare.
In the present case, the best treatment is post-lumpectomy radiation therapy to the breast which is a reasonable and accepted practice.
At present in DCIS, accelerated partial breast radiation is questionable off research protocols. The data in DCIS is young and still evolving and as of yet to be confirmed. The followng options are available:
Generally off protocol we treat the breast to 50 Gy at 2 Gy per fraction or 50.4 Gy at 1.8 Gy/fraction in 25-28 fractions. This is treated either with or without radiation boost. Alternative dose and fraction schemes have been described. Hypofractionated breast treatment in DCIS is presently regarded as usually inappropriate treatment without boost. With a boost, it may be appropriate.
There are no phase III data for DCIS, but a consensus is that most radiation oncologists would boost. A boost of 16 Gy may be higher than necessary with widely negative margins. For ER positive disease, generally, tamoxifen is recommended by the ACR panel. At the latest review, the ACR considers 5 years of tamoxifen usually appropriate.
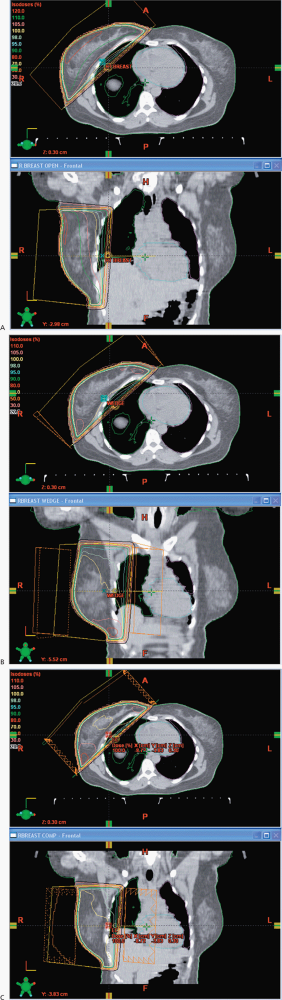
In this case, I would propose a boost of 10 Gy for a total dose of 60 Gy. The primary fields for the whole breast are opposed tangents with the patient on a breast board to obtain good geometry for breast coverage with the arms over the head in a supine position. We use a vac-loc to immobilize the head, arms and upper torso on the breast board with the board elevated to bring the mid sternum parallel to the couch and posterior collimator. We try to avoid, if possible collimator rotations to minimize beam divergence, and half beam block the deep portion of the tangent. The breast is outlined with wires placed at the superior, medial, inferior and lateral borders. The anatomic landmarks include the inferior margin of the head of the clavicle, mid sternum, 2 cm below the inframammary fold and the mid-axilla line. As all chest/breast setups are clinical, we do a control scan in the mid-region of the breast to identify the positioning and verify correctness of the lateral wire, which often requires some adjustment. This will minimize the setup uncertainty. The images are then sent to the treatment planning system for block design, with blocks placed to insure coverage of the breast tissue while minimizing the dose to the lung, heart, contralateral breast, and accounting for breathing motions. Some centers use deep inspiration breath hold technique, but this requires a fast scanner and rapid treatment administration. The boost field is generally given with electrons and if electrons are not suitable due to depth then a reduced opposed tangent field is useful. We do not treat the axilla.
Reviewing the NCCN guideline recommendations concerning treatment, the guidelines allow for lumpectomy without radiation, as category 2B (lower level evidence) which is at varience with the ACR appropriateness criteria which rates lumpectomy alone as "2" usually not appropriate. The NCCN guidelines do rate mastectomy or lumpectomy plus radiation as category 8 and 9, generally appropriate.
Based on the EBCTCG meta-analysis and the individual EORTC and NSABP studies, all have shown a significant reduction in risk of recurrence with post lumpectomy RT, local failures at 20 years up to 1/3 without RT and 15% with RT. In addition the risk of invasive recurrences are much lower with radiation compared with half and half without radiation. The meta-analysis confirmed a 15.5% absolute 10 year risk reduction. No study has demonstrated a change in overall survival.
Ongoing followup includes a 6-12 month interval H&P for 5 years, then annually. A 6 month post radiation mammogram is recommended and then annually thereafter.
The various treatment options are:
Most surgeons in this setting, according to the ACR would proceed to lymphatic sampling using SNB with the mastectomy. She has extensive DCIS in multiple quadrants. There is increasing resistance to SNB/surgical staging of the axilla in recent years.
Lumpectomy with or without axillary staging would result in a poor cosmetic outcome, would reqire radiation therapy and margins would have to be larger to insure no occult disease remains. Therefore, lumpectomy with our without axillary staging would yield unnacceptable outcomes. Poor cosmesis is a relative contra-indication to breast conserving surgery. Likewise, the extent of disease is confirmed by the biopsy and mammogram. An MRI is unlikely to yield additional information, and therefore is supurflous.
The best treatment alternative for this woman is mastectomy, generally with lymph node staging without post-mastectomy radiation.
She has no comorbidities, the US SSA life expectancy tables give her a life expectancy of over 10 years, and due to otherwise excellent health suggest she may live another 12 years. The EBCTCG meta-analysis gives an absolute risk reduction of 15% recurrence at 10 years, and a relative risk reduction of about 50% from both EORTC and NSABP trials. Therefore, there is a significant likelihood she will live long enough to benefit from post-lumpectomy radiatiation therapy. Tamoxifen will reduce her risk of recurrence significantly as well, but many women do not continue on anti-estrogen therapy for long enought. On the other hand, even if she did recur, half of the recurrences are repeat DCIS and those can be managed with mastectomy or repeat lumpectomy.
The ACR scores both of these options a "7" or "Usually appropriate (7-9)"
Therefore, there is no clear answer to the best adjuvant treatment in this setting. To date, all studies have demonstrated a 50% or greater risk reduction, and some authors have reported a 2% risk per year in untreated post-lumpectomy DCIS patients, giving her a life time risk of recurrence of at least 20 - 22%. Radiation can reduce the 11% risk of invasive cancer substantially, making any potential recurrence much more likely to be non-invasive disease. As there are no clear recommendations, a frank discussion with the patient identifying the risks and potential benefits of radiation is necessary.
She elects to proceed to radiation therapy
DCIS is generally treated to the breast only using opposed tangents with or without a boost. As nodal involvement is rare, the axilla does not need to be treated, nor does the supraclavicular fossa. Accelerated partial breast radiation remains unproven and should be used primarily on protocol. There may be cases where APBI may be appropriate off protocol. Treatment options are:
The following dose /fraction schemes are discussed by the ACR and NCCN.
40 Gy is generally deemed to be too low a dose in breast cancer. Treating the breast to 50 Gy or 42.5 Gy (hypofx) is considered reasonable and will give good local control. Boost in this patient population is of uncertain benefit, but is often used in some centers. It is likely that 66 Gy is overkill and is not needed.
My recommendation matches the ACR criteria: Treat to either 42.5 Gy at 2.656/day or 50 Gy at 2 Gy/day. While the ACR feels that the hypofractionated regimen may be less appropriate in younger women, they go on to say in this case, this dose/fraction scheme is ok here by suggesting that hypofractionated data from the NCIC and UK trials can be extrapolated. I would go slightly further to say we probably should be consistent in our extrapolations of data. It is either ok in both cases, or not.
Stereotactic or excisional Biopsy.
She undergoes excisional biopsy (lumpectomy with widely clear margins) revealing a 0.9 cm DCIS comedo type of intermediate nuclear grade. ER is negative.
Adjuvant radiation therapy is recommended in the treatment of DCIS after breast conserving surgery. She has clear margins, so there is no need for re-excision or other additional surgery unless she elects a mastectomy as definitive treatment. Options include (stratified by ACR appropriateness rating):
Nodal staging is not generally needed in DCIS. Some surgeons will proceed to nodal staging if there is a suspicion of higher risk of potential occult invasive disease, such as a high grade disease with comedo-necrosis. In that situation, the ACR awards similar appropriateness ratings to nodal staging. In this setting where there is no clear indication of the potential for occult disease and the lesion is easily sampled comprehensively, the risk of nodal disease is very low (0-5%) and surgical staging is not needed. (Perhaps we could create a new category called statistical staging.)
The best recommendation for further treatment is to proceed to either post-lumpectomy (excisional biopsy) radiation therapy or mastectomy. Both have been shown to significantly reduce the risk of recurrence at 10 years with a 50% relative risk reduction with radiation and a 15% absolute risk reduction over 10 years. Without further treatment her risk of recurrence based on the EBCTCG analysis is 2.1%/year with a life expectancy of 40 years. Further radiation will substantially reduce the risk of invasive recurrences.
She elects to proceed to radiation therapy.
Initial treatment is to the whole breast. Opinion is divided on whether or not to add a boost and what benefit a boost adds. The ACR recognizes this and deems either approach is appropriate. A recent single institution study of younger women with close margins ± boost found a statistically significant indicator of recurrence. (Eur J Surg Oncol. 2013 Jun;39(6):613-8. doi: 10.1016/j.ejso.2013.03.002. Epub 2013 Mar 20.) Accelerated partial breast is only accepted on protocol as appropriate. Possible alternatives include
The ACR feels that a 16 Gy boost is potentially appropriate (6), but may be higher than necessary. They score a 10 Gy boost (on top of 50 Gy) as usually appropriate with a score of 8. ( ACR criteria are 1,2,3 usually not appropriate;4,5,6 may be appropriate; and 7,8,9 usually appropriate.)
The ACR categorization is consistent with my own recommendations: Treat the whole breast to 50 Gy at 2 Gy/fraction using opposed tangents then boost with a direct electron field an additional 5 fractions at 2 Gy/fraction to the lumpectomy cavity + 2 cm margin for a total dose of 60 Gy to the cavity.
 |
Generally a diagnostic mammogram with spot/compression views and possible a stereotactic biopsy or alternatively, an excisional biopsy, with wire localization.
She proceeds to excisional biopsy which demonstrates a 1 cm high grade DCIS with comedo features and a single focus of microinvasion. Receptors were ER positive and margins were widely negative at > 5 mm.
She has high grade disease. Most surgeons would recommend lymph node staging using SNB. She can be treated either with mastectomy or with breast conserving therapy. If she chooses mastectomy she should probably have a sentinel node biopsy, based on ACR appropriateness criteria.
The ACR favors full staging in younger women with high grade disease and this case clearly has a concern as there is microinvasion which changes the staging from DCIS to T1mi and Stage 0 to Stage IA, assuming nodes are negative. There is a 16% risk of nodal involvement.
My recommendation is to proceed to sentinel node biopsy and adjuvant radiation therapy. Observation is not an issue as this disease has already developed an invasive component. Her risk based on NSABP data is at least 25-27% 5 year recurrence. Radiation offers a 60% reduction in risk of recurrence. Although no survival benefit has been shown, the trials were not powered to explore this and there are hints on various retrospective reviews that women with radiation do better than those that do not. There is a clear benefit to PMRT in node positive disease based on the Overgaard data (82b/c) which is not directly relevant to breast conservation surgery in node negative patients, but does give us hints that radiation may do more good than the studies show.
She chooses to proceed with SNB 1/1 nodes positive.
She should proceed to axillary lymph node dissection. Most surgeons will agree with this, but some will cite the ACOSOG Z11 trial. This trial carefully selected very low risk patients. ALND will change radiotherapy management, and many radiation oncologists will opt for increased fields to cover nodes felt to be at risk. This may in the form of high tangents, or addition of supraclavicular fields. The benefit of nodal radiation is less clear if she proceeds to ALND and the sentinel node is the only node positive.
The surgeon performs an axillary dissection which reveals 1/7 LN positive.
Radiation to the whole breast with or without supraclavicular radiation is reasonable. With 7 lymph nodes dissected it is probably reasonable to omit radiation to the axilla, although some would advocate treating as fewer nodes were obtained. If 1/12 nodes were positive, adequate nodal treatment of the axilla by the surgeon mitigates the need for axilla radiation and there is likely no need to treat the axilla.
There are several dose/fraction schemes in common use and commonly advocated. WBRT to 42.5 at 2.67 Gy/fraction in 16 fractions (NCIC), the START A/B (MRC-UK) 41.6 Gy in 13 fractions at 3.2 Gy/fraction, 50 Gy at 2 Gy/fraction. The START A/B trials examined women with T1-3 N0/1 M0 breast cancer over age 18 and concluded this could offer similar outcomes to internationally standardized 50 Gy at 2 Gy/fraction. The START B trial looked at 40 Gy in 15 fractions at 2.667 Gy/fraction and concluded that 40 Gy in 15 fractions is reasonable. The ACR disagrees rating 40 Gy cummulative dose including any boost as too low with an appropriateness rating of 2, its second lowest rating, usually not appropriate. Even in Europe, German centers have not implemented 40 Gy in 15 fractions.
A common approach is to treat to 50 Gy at 2 Gy per fraction generally with 6 MV opposed tangents and to boost the cavity to an additional dose of 10 Gy using either electrons or reduced tangents. I too feel that in many cases unless there is unusually high risk factors 16 Gy is higher than necessary. According to a recently published study from the University of Florence1, close margins of < 1 mm would warrant an increased boost dose to 16 Gy, if a re-excision was not attempted.
Concerning 42.56, I do offer this treatment with the caveat that the data is not as long standing as with standard fractionation, and also mention that all standard arms of present NSABP trials do require a boost bringing the total dose to 52.56 Gy in 21 fractions. There are presently two ongoing trials concerning the need for a boost, the TTROG study (Melbourne) and the RTOG study. No data is as of yet, available.
She is 49, ER+ so yes. for at least 5 years.
This is DCIS, there was no invasive cancer. The Stage is Tis Stage 0. She has a choice: continue with breast conserving surgery with adjuvant radation and if receptor positive, adjuvant tamoxifen. Her alternative is to proceed to a completion mastectomy. The NSABP/NRG and the EORTC have both examined DCIS in this setting which demonstrated radiation reduces the risk of recurrence from about 25% to under 10% and the risk of invasive recurrence from 13.4% to 4%.
She elects a lumpectomy. The pathology is comedo-DCIS. What do you recommend?
What is the pathology, receptors and margin status?
She is ER positive, PR positive, the pathology is comedo-DCIS without necrosis. Unfortunately she has a positive margin.
At this point, I would recommend a re-excision unless the positive margin is at the chest wall and re-excision is not possible. I would also like to see the post-lumpectomy specimen mammogram.
The specimen mammogram is negative. The surgeon agrees to a re-excision. The new pathology shows no residual DCIS. What now?
Radiation to the whole breast is now indicated.
Do you need a node staging?
No. Nodes are very rarely positive in DCIS and unless there is something on exam or imaging, the yield is quite low. Further, nearly all of the Level I axillary nodes will beo covered in traditional whole breast radiation fields.
What radiation dose and technique would you recommend?
The whole breast should be treated to 50 Gy at 2 Gy/fraction. Alternatively, the breast has been treated to 46-50.4 Gy at 1.8 Gy/fraction. While there are studies that do not show benefit to boost, the ACR recommends a boost, although with a less score than for whole breast radiation. Most radiation oncologists recommend boost per the ACR appropriateness criteria, although they are somewhat wishy washy. Her risk factors are close margins, however, that has been cleared by the re-excision. The margins are negative. I would boost 10 Gy at 2 Gy/fraction to 60 Gy to the lumpectomy cavity.
She has a palpable lump confirmed on mammogram. Proceed to either ultrasound guided biopsy if the lesion is seen on ultrasound. Otherwise a stereotactic biopsy with wire localization is required. Depending on her other history, if she has not had a contralateral breast mammogram in more than a year, she should have one.
The lesion is biopsied and found to be DCIS, comedo-necrosis. What do you now recommend?
She has a choice of breast conserving surgery followed by radiation therapy or mastectomy. The treatment outcomes from a cancer cure perspective are equivalent. However, there are individualized psychosocial implications in her choice.
She chose lumpectomy. Pathology shows comedo-necrosis, high grade, and negative margins. What is the next recommendation?
She should receive radiation to the whole breast using opposed tangents, 6 MV, followed by a cavity boost to 60 Gy. The whole breast dose is 50 Gy at 2 Gy/fraction. The boost is reasonable in younger women with higher grade tumors.