Advanced Breast Cancer
Anatomy
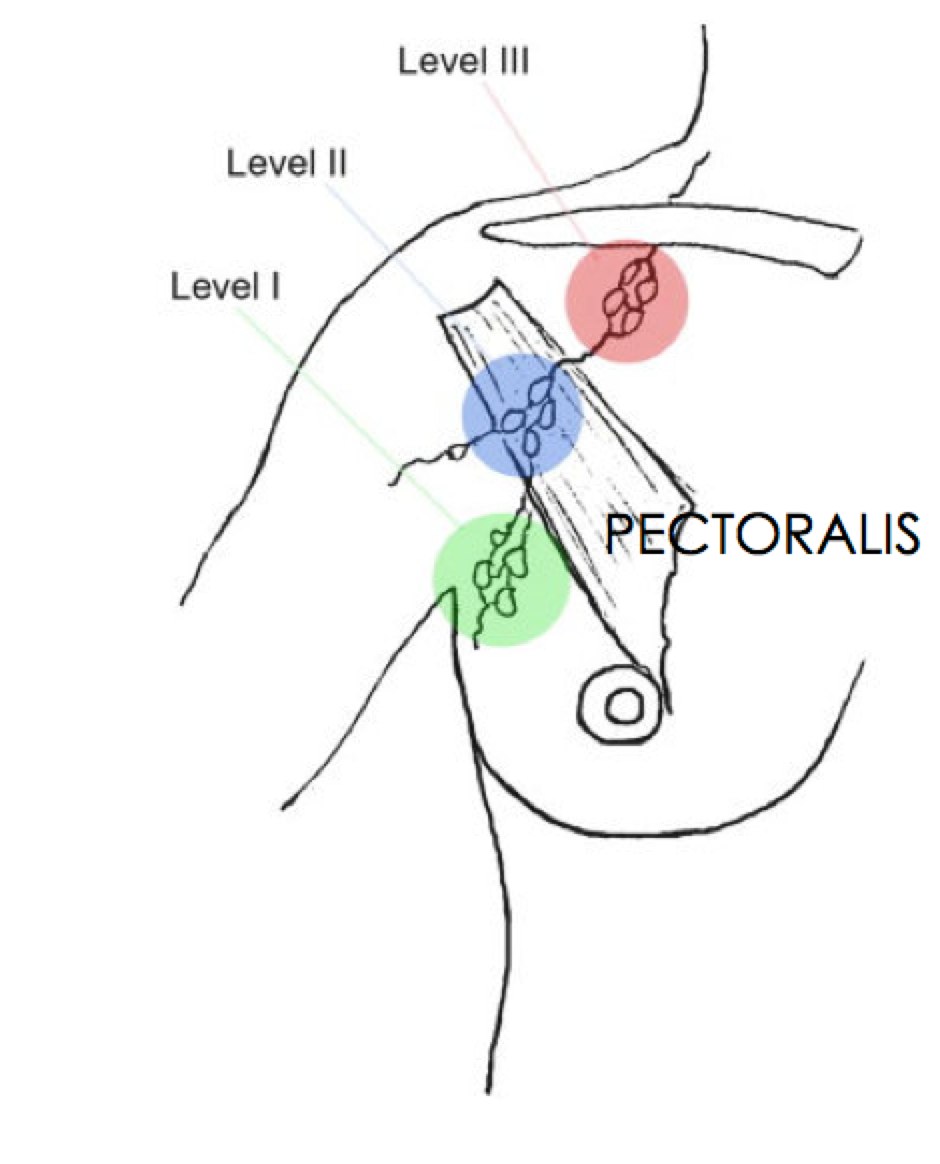
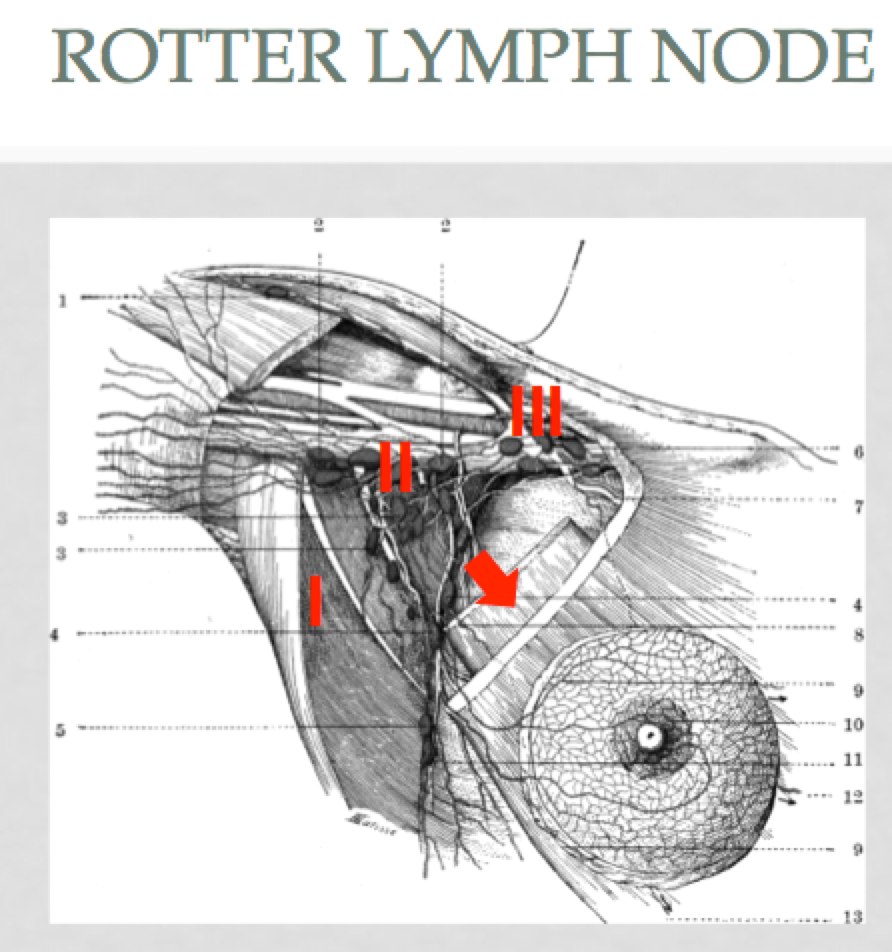
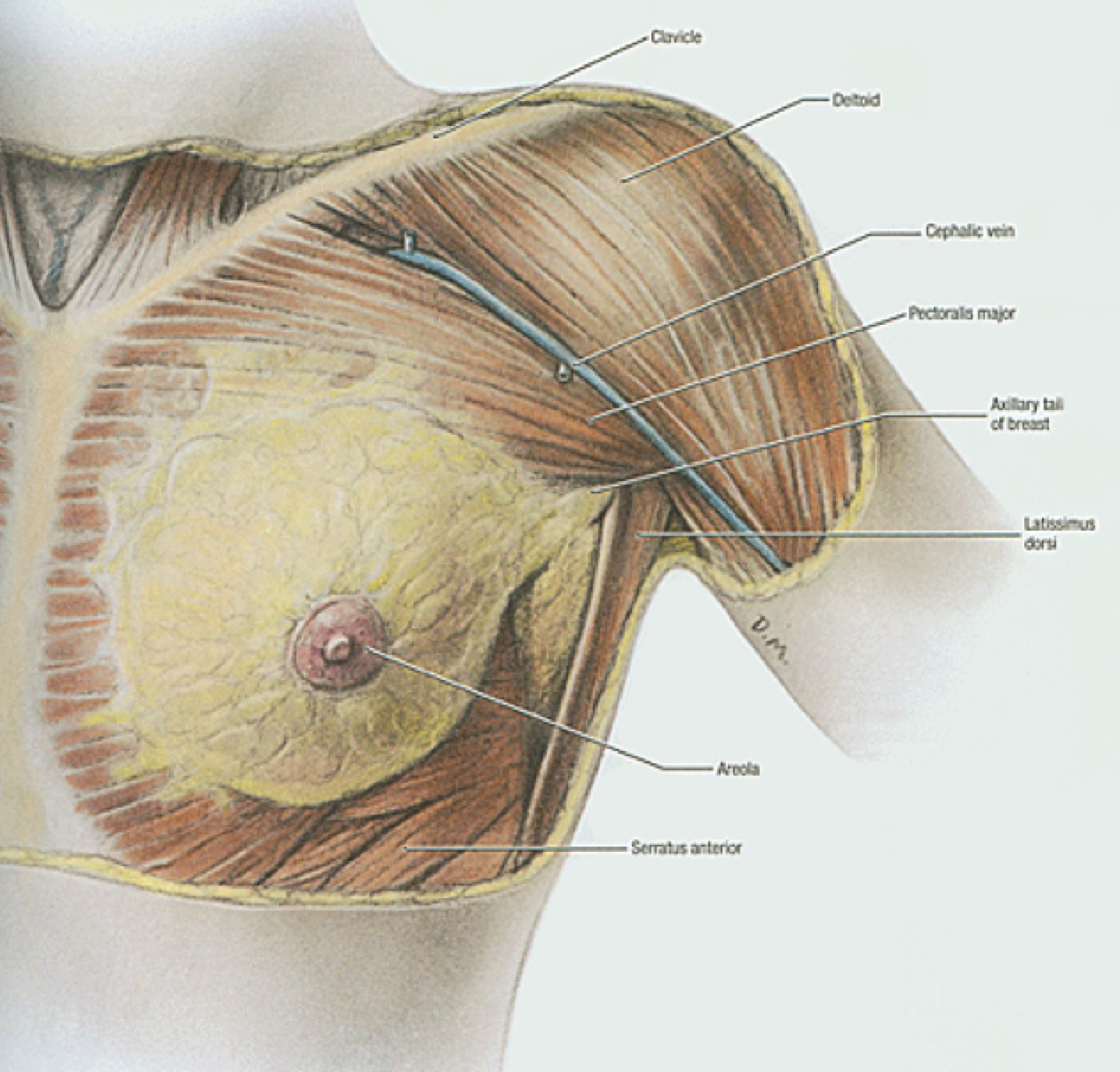
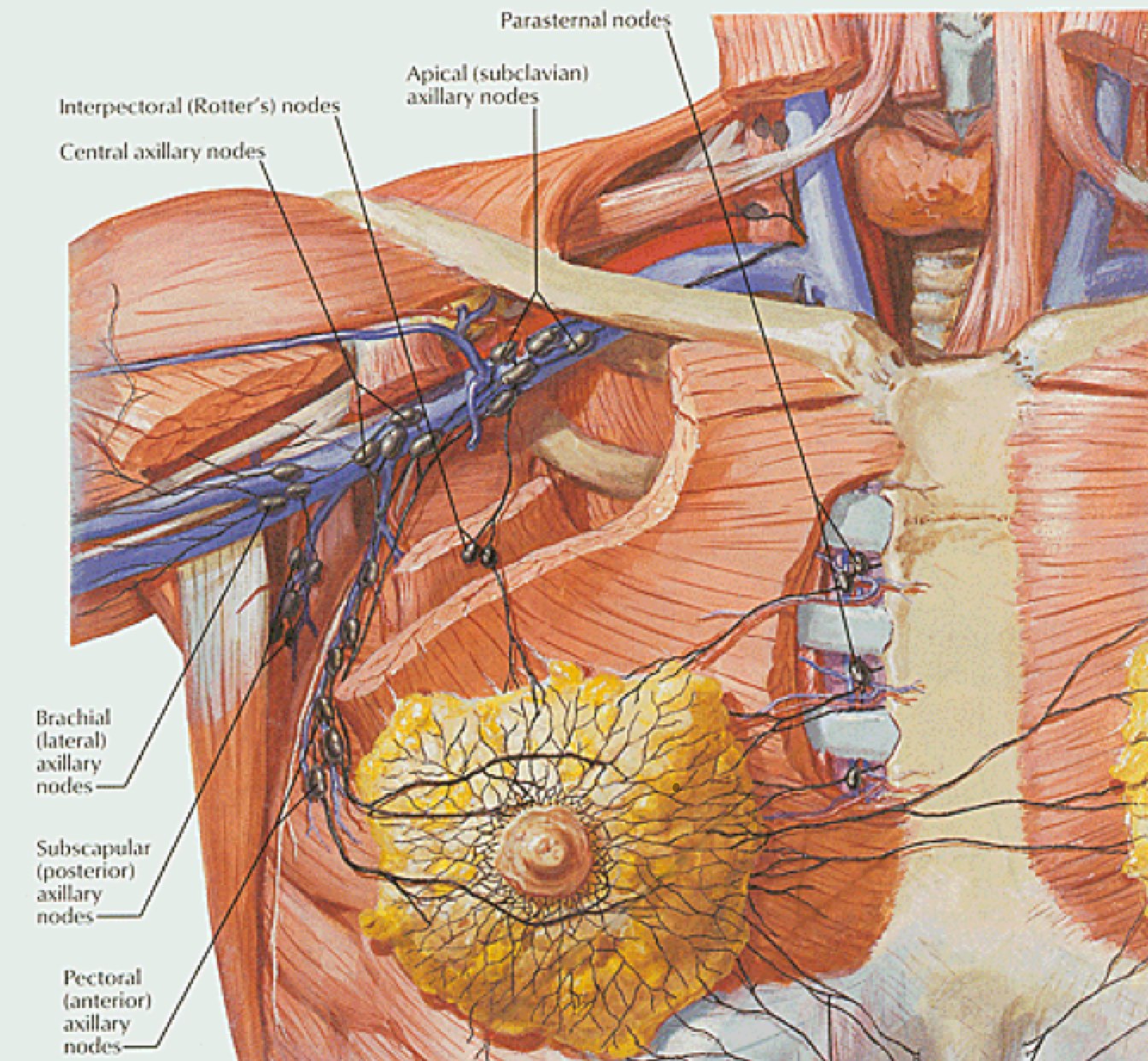
Breast anatomy has been discussed in the Early Breast Cancer section. The additional information here describes the nodal drainages and important surgical landmarks of breast anatomy. Please see Early Breast Cancer for more information.
 |
 |
 |
 |
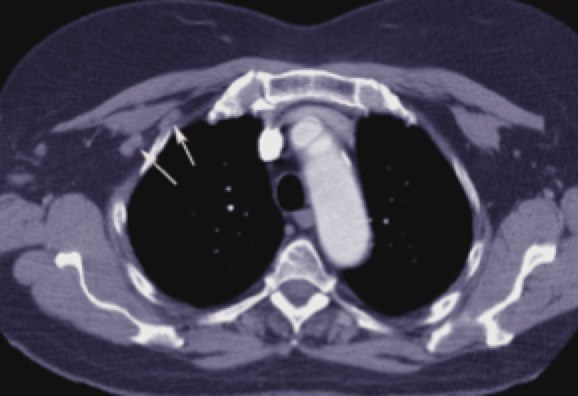 Level 2 ALN Level 2 ALN |
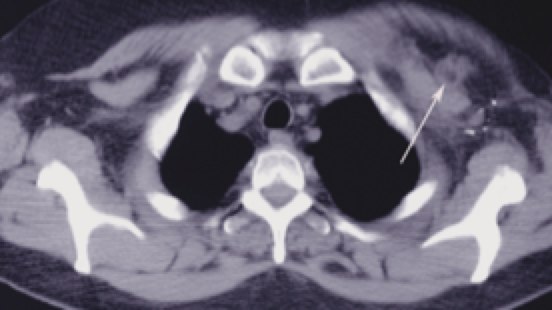 Rotter's Node Rotter's Node |
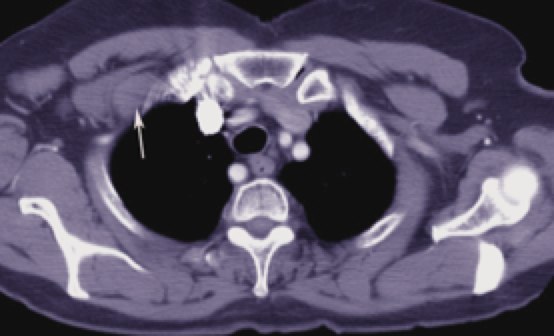 Infraclavicular LN Infraclavicular LN |
Epidemiology
The stage at diagnosis and incidence of breast cancer has been evolving over the past several decades. With the widespread use of hormone replacement therapy, the Women's Health Initiative data leading to the change in HRT practice, the introduction of mammography populaton screening, a stage shift at diagnosis and a demographic shift are other important compounding factors. Encouraging is a trend toward improved breast cancer mortality. Since 2000 the breast cancer incidence rate has decreased by about 3.3%/year and the rate of tomors diagnosed > 5 cm has increased by about 2%/year. Mammography has resulted in an increase in diagnosis during the period 19080-1987, but after 2000, the breast cancer incident rate of tumors < 2 cm has been decreasing by 2%/year. ABout 230,000 new cases of invasive breast cancer were diagnosed in 2011.
Inflamatory Breast Cancer
Inflammatory breast cancer is a locally advanced breast cancer with unique epidemiology, presentation and biology. They are quite rare, involving only about 2%. They have a short time course and about 4000 cases are diagnosed in the US/year. There are no known risk factors, but the disease tends to occur in a younger population. Lymphatic involvement is much more common as are distant metastases. Inflammatory breast cancer is a clinical syndrome characterized by erythema, and edema involving ≥ 1/3 of the breast skin with a palpable border. It can be confused with systitis or mastitis. Dermal lymphatic invasion is not sufficient for diagnosis nor is it required for the diagnosis of inflammatory breast cancer.
Natural History
Prognosis in locally advanced breast cancer has significantly improved. The disease can be cured, and if not cured controlled. As the disease grows in the breast, it may invade the dermis or chest wall. Skin retraction can occur through the invasion of Cooper's ligaments, or invade the lymphatics causing local lymphedema, resulting in peau d'orange. Axillary lymphatic involvement is associated with advanced tumors, with lymph node involvement risk being a function of primary tumor size. Axillary level I → II → III is the general progression thence to the infraclavicular nodes. Additional lymphatic spread is to the internal mammary nodes, but patients rarely fail exclusively in the IMNs. Metastatic spread is also hematogenous, to bone, liver, lungs and brain.
Without treatment, almost all locally advanced breast cancers will progress and become life threatening. Local progression can lead to ulceration of the skin of the breast, pain, bleeding, infection, damage to viscera. Progression of untreated lymphatics can cause pain, brachial plexopathy, edema, obstruction and venous thrombus and skin ulceration.
Treatment advances have improved survival rates for locally advanced breast cancer. Prior to the availability of systemic treatment, mastectomy ± radiation therapy yielded survival rates of 25-45% at 5 years. With the addtion of chemotherapy, 5 year survival rates now approach 80% for Stage IIIA disease and 45% for Stage IIIB disease. Inflammatory breast cancers have a worse outcome than those with non-inflammatory stage IIIB/C. Disease characteristics also make a difference: a long neglected local breast cancer that has not metastasized makes it more likely it can be controlled, and is a favorably prognostic indicator.
Natural History of Inflammatory Breast Cancer
IBC is a clinically diagnosed and distinct category of breast cancer. The key diagnosis criteria are:
- rapid disease onset
- brawny breast induration
- edema (peau d'orange)
- warmth
- assymetric enlargement
- skin erythema extending over a substantial portion of the breast
- extensive lymphovascular invasion by tuor emboli is typically present that involves teh superficial dermal plexus in the papillary and high reticular dermis
- Caution: neglected primary tumors can also lead to breast erythema, edema, warmth and assymetric enlargement, especially with bulk adenopathy.
The disease typically has early metastases and rapid progression but in the United States, 70% have only evidence of local-regional disease at the time of diagnosis. The prognosis has improved with the availability of combined modality therapy.
In the US, about 70% of inflammatory disease is localized when diagnosed. While a worse prognosis disease, significant progress has been made in treating inflammatory breast cancer. Prior to chemotherapy, patients treated with surgery and/or radiation had survival rates of 5% at 5 years. The expected median surival time was < 15 months. Local recurrence rates were high at 50%. The addition of adriamycin based chemotherapy improved outcomes. Currently local control for patients treated with chemotherapy → surgery ⇒ radiation therapy approaches 70 - 80% and OS5 ranges around 40%.
Clinical Workup and Evaluation
Locally advanced breast cancer is usually detected after a palpable mass develops within the breast. Advanced disease can cause local or regional pain, bleeding, parasthesias and paresis. When working up a locally advanced breast cancer, the time couse is important. Inflammatory breast cancer has a very short clinical time course, while a very long course is more reflective of a neglected breast cancer.
Clinical Workup and Staging
A careful history paying careful attention to the time course of the development of symptoms is important. The breast physical examination should note the breast, symmetry within the breast and between breasts. Inspection of the skin for peau d'orange, inflammatory changes and the extent of inflammatory changes, breast retraction and nipple involution. The breast should be palpated to determine the extent of disease. Fixation of the mass ot the chest wall should be determined.
STAGING
All cases of locally advanced breast cancer require a full staging workup to determine T stage, N stage and M stage. Stage III cancer can be present with T3 disease (tumors > 5 cm) with nodes positive, or N2/N3 disease or stage T4.
Stage T4 disease may invade the chest wall (T4a), the skin of the breast or satellite nodels (T4b) or both (T4c). Inflammatory breast cancer is stage T4d. Invasion of the pectoralis muscle without chest wall or skin invasion is not T4 disease.
All cases of locally advanced disease require a comprehensive staging work up before therapy is initiated. Laboratory studies (hemogram, chemistries, LFTs), imaging incluiding CXR, CT abdomen and pelvis, bone scan and additional imaging as needed. Up to 35% of patients with locally advanced breast cancers (clinical Stage III) will have bone scan abnormalities. If neurological symptoms exist, then an MRI of the brain or contrast enhanced high resolution CT scan are necessary.
General Management and Treatment
Neoadjuvant chemotherapy is recommended for most patients with locally advanced breast cancer. The initial extent of disease should be well characterized prior to commencing therapy. Pre-treatment initial extent of disease will guide subsequent treatments and therapy. This initial extent of disease will only be known from initial history and physical exam, and imaging findings. It is imperative that all patients be properly clinically staged prior to initiating treatment.
Locally advanced breast cancer is categorized into operable or inoperable disease. Presently, neoadjuvant chemotherapy is the initial therapy for all inoperable breast cancers. About 80-90% will show response to chemotherapy and most will become candidates for surgery post neoadjuvant chemotherapy. No specific criteria determine operable v. inoperable disease. Generally most feel that if surgery is unlikely to obtain clear margins, then neoadjuvant chemotherapy should be used in an attempt to gain control of disease to permit surgical management with clear margins. This includes most, if not all patients with T4 disease and all patients with inflammatory breast cancer. For other patients with operable Stage III (T4a-c or N2-3) disease surgery followed by chemotherapy is reasonable and is probably equivalent to neoadjuvant chemotherapy.
Neoadjuvant Chemotherapy
The use of neoadjuvant chemotherapy has some advantages over traditional surgery → chemotherapy → radiation therapy course. Several trials have demonstrated neoadjuvant chemotherapy substantially reduces the size of primary tumor and LN metastases in > 80% of all cases. For patients with large primary tumors, using neoadjuvant chemotherapy has been shown to increase the possibility of breast conserving surgery. For patient who have a less ideal response, the possibility of second line chemotherapy may be useful.
Early chemotherapy in locally advanced breast cancer alone has not improved survival. NSABP B18 randomized neoadjuvant v. adjuvant chemotherapy and surgery in operable breast cancer. At 16 years the OS and DFS were the same between the two groups. A second problem with neoadjuvant chemotherapy is the role of lymph node staging. The B18 trial demonstrated clearance of nodes in 20-40% of the patients treated with neoadjuvant chemotherapy, giving rise to concern that SN may clear selectively and earlier. If the nodal stage is unknown prior to neoadjuvant chemotherapy, then radiation therapy field determination becomes more problematic, as well as the decision to offer radiation in post-mastectomy patients where nodal stage prior to treatment is unknown or indeterminant. On the other hand, if neoadjuvant chemotherapy clears lymphatics, then an axillary node dissection would not be done, thus sparing the patient of an axillary dissection.
Neoadjuvant chemotherapy as a strategy to convert large tumors for breast conservation must be carefully considered. Only 23% of post-mastectomy specimens had characteristics that might predict for favorable outcomes in BCS. Important criteria include:
- resolution of skin edema
- favorable clinical response to neoadjuvant chemotherapy
- lack of multicetnricity
- lack of extensive lymphovascular space invasion
Surgery
Mastectomy continues to be the primary local-regional treatment for locally advanced breast cancer in the US.
- Lumpectomy: removal of the primary tumor with a margin of tissue
- Simple or Total Mastectomy: removal of the breast but not the axillary contents
- Modified Radical Mastectomy: removal of the breast plus an axillary Level I/II dissection
- Radical Mastectomy: Removal of breast, pectoralis major and axillary Level I/II dissection
- Extended Radical Mastectomy: Remove of breast, pectoralis m., axillary I/II dissection, IMN dissection ± ALND Level III
- Skin Sparing Mastectomy: Total or Modified radical mastectomy with preservation of native skin for immediate reconstructiond
The key studies B-18 and EORTC that offered neoadjuvant chemotherapy for intended breast conservation enrolled 60% who were likely breast conservation candidates prior to entering the study, thus potentially skewing the results. Breast conservation rates of 60 - 68% showed that 20% of initial mastectomy candidates could have gone directly to breast conservation instead of neoadjuvant chemotherapy. Both B18 and EORTC showed similar recurrence risks with neoadjuvant chemotherapy v. adjuvant chemotherapy.
A meta-analysis examined omitting surgery with neoadjuvant/adjuvant chemotherapy and radiation alone. These findings demonstrate that patients with complete responses will still benefit from surgery in addition to radiation.
Post Mastectomy Radiation Therapy
PMRT has been used for decades. Early trials used radiation and have demonstrated the effectiveness of radiation. Radiation offers substantially improved local-regional control rates for most patients with non-invasive or early stage cancers, including node negative disease. Patients with Stage III breast cancer (≥ 4 nodes positive T3/T4 disease) have a clinically relevant risk of recurrence. Radiation in this setting offers not only a local control advantage but also a survival advantage. For many years PMRT was routinely offered to patients with 4 nodes positive, but there has been controversy over the need for PMRT in 1-3 nodes positive. It is clear that the local recurrence risk reduction with PMRT in 1-3 nodes positive remains, but what is less clear is whether or not this translates into a survival advantage.
The EBCTCG did a comprehensive meta-analysis examining PMRT. Most recently they examined 9933 patients demonstrating PMRT reduced the risk of LRR15 in node positive patients from 29% to 8%. This led to a 5% decrease in the 15 year breast cancer specific mortality from 60% to 55%. A more recent analysis subdivided the cohort into pathologic node status.
| Node | LRR Recurrence without → with Radiation |
|---|---|
| pN0 | 5.8% → 2.4% |
| 1 - 3 Nodes Positive | 24.7% → 5.3% |
| ≥ 4 Nodes Positive | 40.6% → 12.9% |
With the improvement in local-regional control there is an associated statistically significant improvement in breast cancer deaths and overall survival for patients with 1-3 and ≥ 4 nodes positive. In other words, there is a survival advantage gained by adding PMRT to any node positive disease. While there is a local regional control advantage in node negative disease, there is no apparent increase in survival and the relative rate of recurrence is low enough that there is not an appreciable advantage to adding radiation in the post mastectomy setting in node negative patients. Older studies using older non-conformal 3D techniques did cause greater treatment related morbidities with obsolete radiation fractionation schedules. When these studies are excluded, results improve even more. Adjuvant radiation therapy probably improves survival further, even with the use of systemic therapy to reduce the risk of distant metastases. Whelan's PMRT meta-analysis demonstrated an substantial reduction in the risk of any recurrence with an odds ratio of 0.69 and a survival advantage with an OR of 0.83. When radiation therapy quality was considered, which was defined as an optimal dose of 40 - 60 Gy at 2 Gy/fraction and optimal field arrangements that include both the chest wall and regional lymphatics, the proportional risk reduction for LRR was 80% (optimal dose/fractions/fields) compared with 70 - 65% with sub-optimal treatment. There was a statistically significant improvement in breast cancer mortality in the trials that used optimal radiation dose and treatment fields but not in other trials.
A number of key studies of PMRT habe been performed. Most notably, the Danish 82b/c and Vancouver studies demonstrated continued advantages of PMRT with the addition of systemic therapy. These studies have been criticized for having a poorer performance in the standard arm and the Danish studies in particular for less than optimal axillary node dissection. However, Marie Overgaard did a subset analysis, examining data restricted to patients with ≥ 8 nodes. In this subset, the advantages of PMRT remained in node positive breast cancer. It was also thought that a less than optimal ALND would increase the risk which radiation will mitigate. The Danish studies demonstrated a risk of recurrence rising at 1% per year between 10 and 25 years of follow up.
Tumor size is also an important factor. MDACC uses ≥ 4 cm as an indication for PMRT and the IBCSG found that pre-menopausal women with 1-3 nodes, Grade 2-3 disease with vasculary invasion, had risk factors from 19% - 27%. Margin factors were also an important risk factor.
These findings are summarized from the International Breast Cancer Study Group Trials I - VII.
| Premenopausal Women | ||
|---|---|---|
| Local regional recurrence rate 19% - 27% | |
| Local regional recurrence rate <15% | |
| Postmenopausal Women | ||
|---|---|---|
| Local regional recurrence rate 24% | |
| Local regional recurrence rate < 15% | |
Margin status is also a consideration. In patients with 0 - 3 positive lymph nodes and close or positive margins with tumors < 5 cm and no adjuvant radiation had a relatively higher risk of local recurrence. The recurrences are reported at around median interval of 26 months with an 8 year incidence of 18%. For women ≤ 50 the rate was 28% v. 0% for women > 50. A second investigator (Katz) found close/positive margins predicted for recurrence independently of age at 10 years with 45% in this cohort, 33% in those who have pectoral fascia involvement even if negative margins are achieved.
There are presently Asian and European trials accruing to examine further the role and indications for PMRT. Radiation continues to show a significant benefit in risk reductions of 20% to 40% for local regional recurrence rates.
PMRT after Neoadjuvant Chemotherapy
PMRT after neoadjuvant chemotherapy brings new questions to when PMRT radiation should be offered. NAChT changes the pathologic staging, and thus information we have previously relied upon to make radiation decisions is no longer available. Information is limited. One study compared 579 patients treated with neoadjuvant chemotherapy → mastectomy → radiation therapy to 136 patients treated with chemotherapy → surgery alone. Radiation was given at the discretion of the treating physician and patient preference.
Patients treated with post-neoadjuvant chemotherapy/mastectomy → PMRT had generally worse disease characteristics. Despite this local-regional recurrence was significantly lower in the group treated with PMRT than with chemotherapy and surgery alone. Multivariate analysis demonstrated that the addition of radiation in Stage III breast cancer or extensive residual disease after chemotherapy was independently associated with decreased risk of local-regional recurrence and decreased breast cancer mortality (HR=7.0 in favor of radiation).
A study by the same group looked at Stage II (T3N0) disease and found that patients in this group had a post-chemotherapy recurrence rate of 24% and a post radiation (after neoadjuvant chemotherapy) recurrence rate of 24%.
Two ongoing studies are newly established (2014) to look at the role of PMRT in locally advanced breast cancer post neoadjuvant radiation therapy: The Alliance Cooperative Group (ACOSOG/CALBG/NCCTG) and the NRG (NSABP/RTOG/GOG) have each established studies to determine the role of radiation in the neoadjuvant chemotherapy setting.
The NRG study is accruing patients with cT1-3N1 disease treated with neoadjuvant chemotherapy who convert to ypN0 ± RT to comprehensive nodal RT v. no nodal radiation. The Alliance study examines patients with cT1-3N1 disease treated with neoadjuvant chemotherapy who are sentinel node biopsy positive randomized to completion ALND or axillary radiation. All patients will receive chest wall radiation.
Radiation Therapy Treatment Planning And Techniques
Techniques and doses are as for local disease. Generally the draining lymphatics are treated comprehensively, either surgically or with radiation. (Breast Cancer field setup) Post mastectomy radiation included treatment to the chest wall, draining lymphatics and the undissected axillary apex and supraclavicular fossa. Stage III (T3N1, T4 or pathologic N2-3) disease have a clinically relevant risk of axillary apex or supraclavicular recurrence. A study of > 1000 patients with N2 (≥ 4 nodes positive) disease, 20% or greater nodes positive (MDACC-"nodal ratio" study) or ECE > 2 mm treated with mastectomy → chemotherapy without radiation found recurrence rates at 10 years in the axillar apex/SCF of 14% - 19%.
For Stage I/II, PMRT benefit is less clear. For patients with large number of axillary nodes dissected (average 17), LRR10 is 3% and not predicted by extent of dissection, or ECE. The Danish 82b/b studies demonstrated 43% of the no radiation arm patients who developed local-regional recurrence had an axilla recurrence as a component of their recurrence. It has been suggested that this recurrence rate was a result of less extensive surgery in the axilla with a median number of nodes recovered of 7 (compared with 17), thus the suggestion that the need to include Axillary Level I/II LN in the PMRT target may be determined by the completeness of the ALND. With this theory, the ALND itself is therapeutic.
Internal Mammary Nodes
Aside from the 1-3 nodes controversy, the need to treat the internal mammary nodes is highly controversial and is now the subject of ongoing Phase III studies. The justification for treating IMN is based on older experiences dissecting IMN chain. Up to 35% - 50% with clinically advanced disease, will have micrometastases in the IMNs. A more recent study from China showed rates of IMN involvement as follows:
| Subgroup | IMN Involvement Rate |
|---|---|
| 4-6 ALN+ | 28% |
| ≥ 7 ALN+ | 42% |
| Medial Tumors and 1-3 ALN+ | 24% |
| Medial Tumors and 4-6 ALN+ | 48% |
| T3 and age ≤ 35 years | 25% |
| T2 and 1-3 ALN+ | 20% |
| T2 and 4-6 ALN+ | 32% |
| T2 and ≥ 7 ALN+ | 42% |
| T2 medial tumors | 21% |
From this Chinese surgical series, the greater the number of involved axillary nodes, appears to be one of the more important factors in predicting rate of IMN involvement, but in each of the cases in this series, the involved IMNs is > 20% which is often used as a standard in other disease sites for adding lymphatics to treatment fields.
Failure analysis studies demonstrate the risk of failure in the axilla and supraclavicular fossa with 1-3 nodes positive, no extracapsular extension and Stage II breast cancer is 4%. Despite this, trials showing survival advantage (Danish 82b/c, MA-20) have treated the axilla Level III and supraclavicular nodes within the treatment fields. This suggests there may be benefit of treating the lymphatic drainage in Stage II disease.
Fields and treatment volumes
CT simulation is very useful to precisely delineate the target volumes. The IMN vessels are found within the first 3 intercostal spaces and are the region where the IM nodes are at highest risk. Using the IM vessels. The depth of the Level III axillary and supraclavicular nodes is also variable. The depth can be easily determined from CT imaging. Contouring these nodes in the treatment planning system can insure they are adequately treated. Studies of treatment planned patients using a 6 MV anterior oblique supraclavicular field prescribed to a depth of 3 cm can significantly underdose nodes in patients who are overweight or obese.
Technique and Setup
Patients should be immobilized with their ipsilateral arm abducted to between 90° and 120° and externally rotated. The goal is to insure that the soft tissues of the arm are clear of the superior edge of the tangent fields and this position will also help clear skin folds in the axilla. The neck should be turned away from the field to attempt to minimize skin folds in the supraclavicular fossa.
An elevated "breast board" is used to position the anterior chest wall such that the slope of the sternum is parallel to the table top.
Radioopaque wires are placed on the surgical scar and at the perimeters of the former breast tissue. The upper wire is placed at the bottom of the head of the clavicle. This wire separates the supraclavicular field from the tangents. The inferior wire is placed 2 cm below the pre-mastectomy inframammary fold. This can be estimated from skin texture changes and comparing to an intact contra-lateral breast. This position becomes more of a clinical judgement decision in the event bilateral mastectomies have been performed. The medial wire is in the midline of the sternum and the lateral wire is approximately in the mid-axillary line. This lateral wire will vary based on individual anatomy. The rough position should allow the tangent fields to be just anterior to the latissius dorsi.
Once the localization wires are placed, and the patient is established and immobilized the the treatment position, CT imaging (scout and control slices) will help determine if the setup is reasonable and the wires are placed well using both the anatomic landmarks and the CT imaging landmarks. Then a final CT planning scan is obtained for contouring areas of interest to insure coverage of required areas, while minimizing dose to normal tissues.
Several techniques have been used to treat the chest wall and internal mammary fields. These techniques are:
- Tangents with matched oblique 15-25° electrons
- Three en face electrons with junction feathering every 8-10 Gy (weekly)
- Partial wide tangents
The first technique entails setting the supraclavicular field with the isocenter at the predetermined match line at the bottom of the head of the clavicle. The gantry is rotated away from the breast about 5-10° to avoid the spinal cord and the lower portion is half beam blocked to prevent divergence into the tangent field. This is important to insure the matched point dose not underdose or overdose the chest wall, resulting in rib injury or increased risk of recurrence. The tangent angles are set by rotating the gantry to align the breast marker wires on the simulation CT and half beam blocking the inferior half at the isocenter to avoid deep divergence into the chest. An alternative to half beam blocking is to overrotate the gantry so that the deep edge of the beam is non-divergent. This technique is not often necessary with modern dual independent jaw accelerators.
The superior edge of the tangents must be matched with the supraclavicular field inferior margin. The easiest and safest way to match is to use a single isocenter technique in which the tangents are half beam blocked at the superior half of the field and at the deep half of the field. This will assure a match line. There are two problems with this approach: 1.) the inferior divergence of the tangent is doubled, resulting in more superficial abdominal dose and 2.) some patients have a long chest resulting in the accelerator half beam distance being too short for full coverage. In this case separate isocenters must be used and the upper border matched by rotating the table to match. Most treatment planning systems are capable of calculating this match line. If not there are a number of techniques to set the match divergence which will compute a table rotation to match the beam divergence. The foot of the table will be rotated away from the gantry to insure the diverging tangent matches the non-divergent inferior edge of the anterior oblique supraclavicular field. The relationship of the table is (θ)=tan-1[0.5*(tangent field length/SAD]. A typical value is around 7°
Recently a review of treatment planned cases examining good coverage to comprehensive nodes, while minimizing dose to uninvolved tissues has been reported. This study concluded that partial wide tangents offered the least volume of normal tissue irradiation while maximizing probability of full coverage of at risk areas.
Dose and Fractionation
Initial fields and volumes are treated to 50 Gy at 2 Gy/fraction.
Outcomes, Patterns of Failure, Prognostic Indicators
In recent years there has been significant improvement in prognosis. Previously the disease was characterized by higher reates of local-regional recurrence, distant mets and death. Advances in all disciplines (surgery, chemotherapy, radiation) have improved teh outcomes of locally advanced patients. 10 year local regional control rates of 89% have been reported. Patients with ER negative disease, significant residual skin invasion had LRR rates approaching 20%. A reasonable OS10 estimate for stage III disease is 50%.
Supraclavicular node disease is an adverse prognostic indicator, but some studies report OS10 rates of 25%, and local control rates of 77% at 5 years and and OS10=47%. Patients who had SCN disease with CR to chemotherapy did better than those who had persistent disease.