Hodgkins
Anatomy
Epidemiology
There are about 7,500 new cases of Hodgkin's Lymphoma in the US each year. There is a bimodal distribution with peaks at age 25 years and at age 60 - 70 years. The second peak of the curve is much smaller than the earlier peak. Most of the disease occurs in younger patients. A number of studies have suggested a genetic predisposition for HL with a note of increased incidence in Jews and amoung first degree relatives.
Pathology
There are two broad categories of HL: Classic (more common) and Nodular lymphocyte predominant HL (NLPHL).
Classic Hodgkins Lymphoma has four subtypes:
- Nodular Sclerosing (most common type in US)
- Mixed Cellularity
- Lymphocyte depleted (worst prognosis)
- Lymphocyte rich (best prognosis)
Classical Hodgkin Lymphoma (CD15 pos/CD30 pos/CD20 neg) is divided, for treatment purposes into three groups:
- Early Stage Favorable
- Stage I/II without Risk factors
- Risk factors are defined differently by different groups: GHSG, EORTC, and NCI-C. (see Risk table below.)
- Early Stage Unfavorable
- Stage I/II with Risk Factors
- Advanced Hodgkin Lymphoma (Stage III/IV)
Risk factors associated with Hodgkin Lymphoma:
| Factor | GHSG 2010 | EORTC | NCIC |
|---|---|---|---|
| Number of Nodal Sites | ≥ 3 | ≥ 4 | ≥ 4 |
| ESR/B-Symptoms | ESR ≥ 50: no B signs ESR ≥ 30 "B signs" | ≥ 50, no B signs ≥ 30 with B signs | ESR ≥ 50 |
| Extranodal Disease | Present | Present | |
| Age | > 50 y | ≥ 40 y | |
| Histology | MC/LD | MC/LD |
The key conditions for German GHSG that identify FAVORABLE HL are: 1-2 sites of involvement, No large mediastinal adenopathy (defined as < 1/3 of the transverse diameter of the chest), No extranodal involvement, and a Favorable ESR/B-signs profile, namely, ESR ≥ 50 without B symptoms or ESR ≥ 30 with B symptoms.
Natural History
Clinical Workup and Evaluation
PET/CT has long been used to determine the extent of disease and response to treatment. More recently, there have been several attempts to quantify the response to therapy. These included the RECIST criteria and the Revised RECIST Criteria (CR=complete response, PR=partial response, SD=stable disease, and PD=progressive disease).
| CR | Complete Response | Disappearance of all target disease: pathologic LN reduced to < 10 mm. |
| PR | Partial Response | At least a 30% reduction in the sum of diameters of target lesions |
| SD | Stable Disease | Insufficient shrinkage to qualify for PR and insufficient growth to meet PD criteria |
| PD | Progressive Disease | A relative increase of ≥ 20% of the sum of diameters of disease |
More recently, a response criteria based on PET/CT has discussed and incorporated into the NCCN guidelines with rapid adoption, and dissemination. These criteria are called the Deauville Criteria and they essentially describe what practicing clinicians have long used to identify PET response: Using the liver metabolic uptake SUVs as a reference point, along with the mediastinal blood pool metabolic uptake SUVs, the relative response is divided into 5 criteria now called the Deauville Criteria. The big problem with this Deauville Criteria model is that most practicing radiologists skilled in PET interpretation do not report this and have not been trained. It too is a relatively subjective criteria, but attempts to further quantify the PET SUV data. Whether this will have significant impact or not is, at the moment, questionable, but for the moment, anyway, we should be aware of it. So, here goes.
| Deauville | Definition |
|---|---|
| 1 | No 18FDG uptake above background |
| 2 | 18FDG uptake ≤ mediastinal blood pool |
| 3 | 18FDG uptake > mediastinal blood pool and ≤ liver |
| 4 | 18FDG uptake increased compared to liver |
| 5 | 18FDG uptake markedly increased compared to liver |
The Deauville Criteria are broadly divided into "Negative" (1-3), and "Positive" (4-5) for the purposes of determining further therapy paths. This division clearly and directly matches what many clinicians have long used for decision making criteria based on PET: Is it more metabolically active than the liver or not. It remains unclear what prognostic or directive value these newly named criteria will offer.
- Negative
- No uptake above background SUV
- Uptake ≤ mediastinal blood pool
- Uptake > mediastinal blood pool but less than liver
- Positive
- Uptake moderately increased compared to liver
- Uptake markedly increased compared to liver
General Management and Treatment
Early Favorable Hodgkin Lymphoma
There are two treatment modalities that are used: Chemotherapy and Chemotherapy followed by radiation. To date, all studies have demonstrated an advantage of adding radiation therapy. The risk for recurrence is decreased with the addition of radiation to chemotherapy by at least 60%. (If the risk of recurrence is 20%, post chemotherapy, then radiation will reduce that risk to 10%). All Stanford V regimens used post-chemotherapy radiation therapy.
The German GHSG HD10 for Stage I/II (favorable) randomized to ABVD (adriamycin, bleomycin, vincristine and dacarbazine) x 4 cycles v. ABVD x 2 cycles followed by either 20 Gy or 30 Gy of IFRT. No PET imaging was performed. ABVD x 2 v. ABVD x 4 showed no difference in outcomes. An article in the JCO (JCO 2015;33;625) looked at the NCDB and over 20,000 patients. The authors found that combined modality therapy was associated with a survival advantage, but salvage was 30% in relapse, so the overall absolute benefit was not particularly large at 4%, but there is a survival benefit at HR 0.6 in favor of radiation.
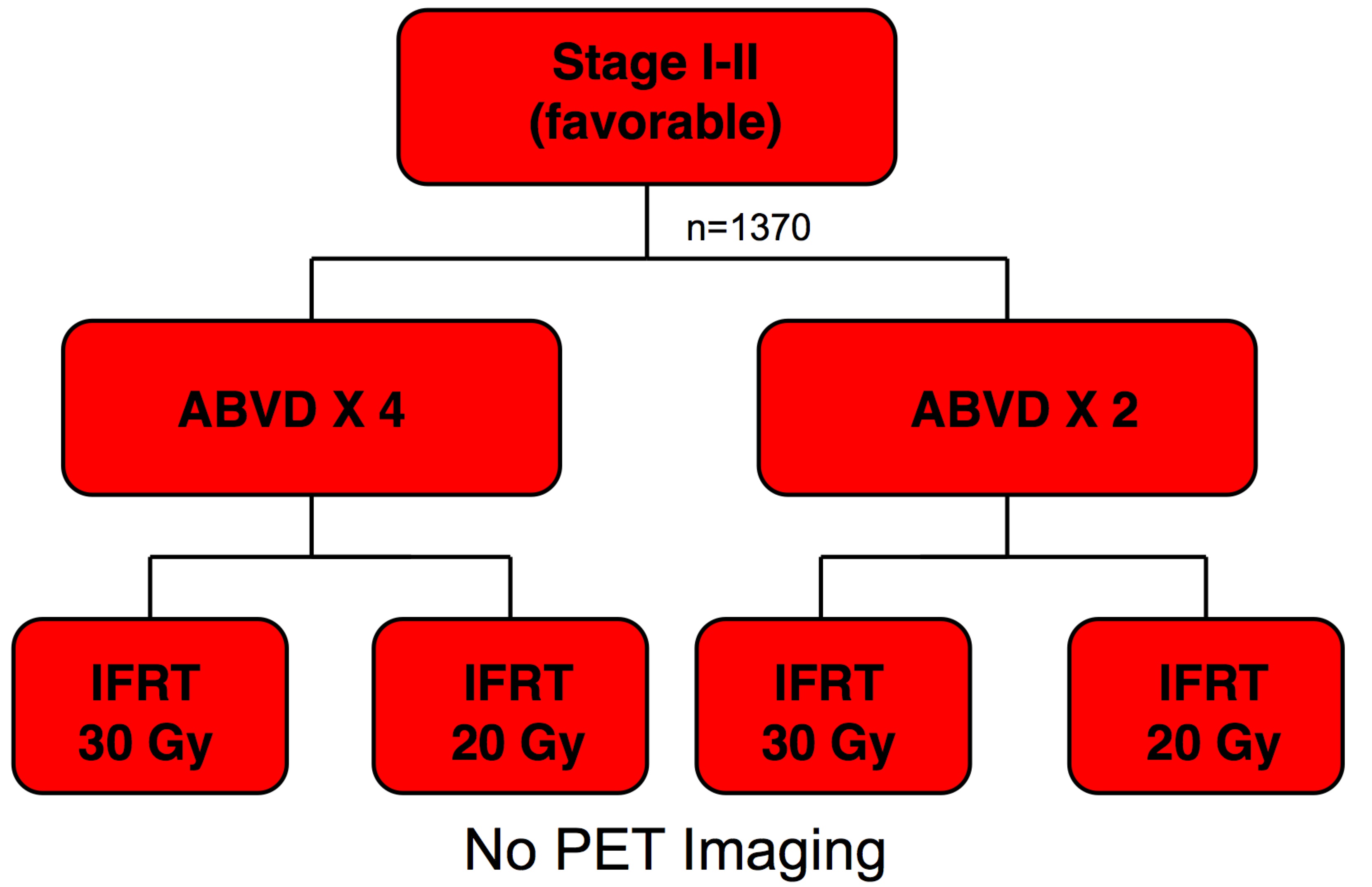
For early stage, Favorable HL, there is no demonstrated benefit for more than 2 cycles of ABVD. The recommended dose for patients without "B" signs is ABVD x 2 cycles followed by ISRT to 20 Gy.
For "favorable," all favorable criteria must be met, namely, No B-signs, 1-2 sites, no bulky/large adenopathy, no extranodal disease, favorable ESR (≤ 50 or with B-signs, ≤ 30, but this is very rare)
Unfavorable Hodgkin Lymphoma
Unfavorable early Hodgkin Lymphoma is defined as any disease that is bulky (> 1/3 mediastinum, or > 5 cm - 10 cm), more than 3 sites of involvement, extranodal involvement, or unfavorable ESR profile). For HL to be considered unfavorable, any of these conditions present make the disease "unfavorable."
The German GHSG studies for unfavorable HL, Stage I/II looked initially at BEACOPP and ABVD and 20 or 30 Gy of radiation. The initial study (Eich et al, Heidelberg, JCO 2010;28:4199 raised doubts about BEACOPP and concern that 20 Gy might be too low a dose.
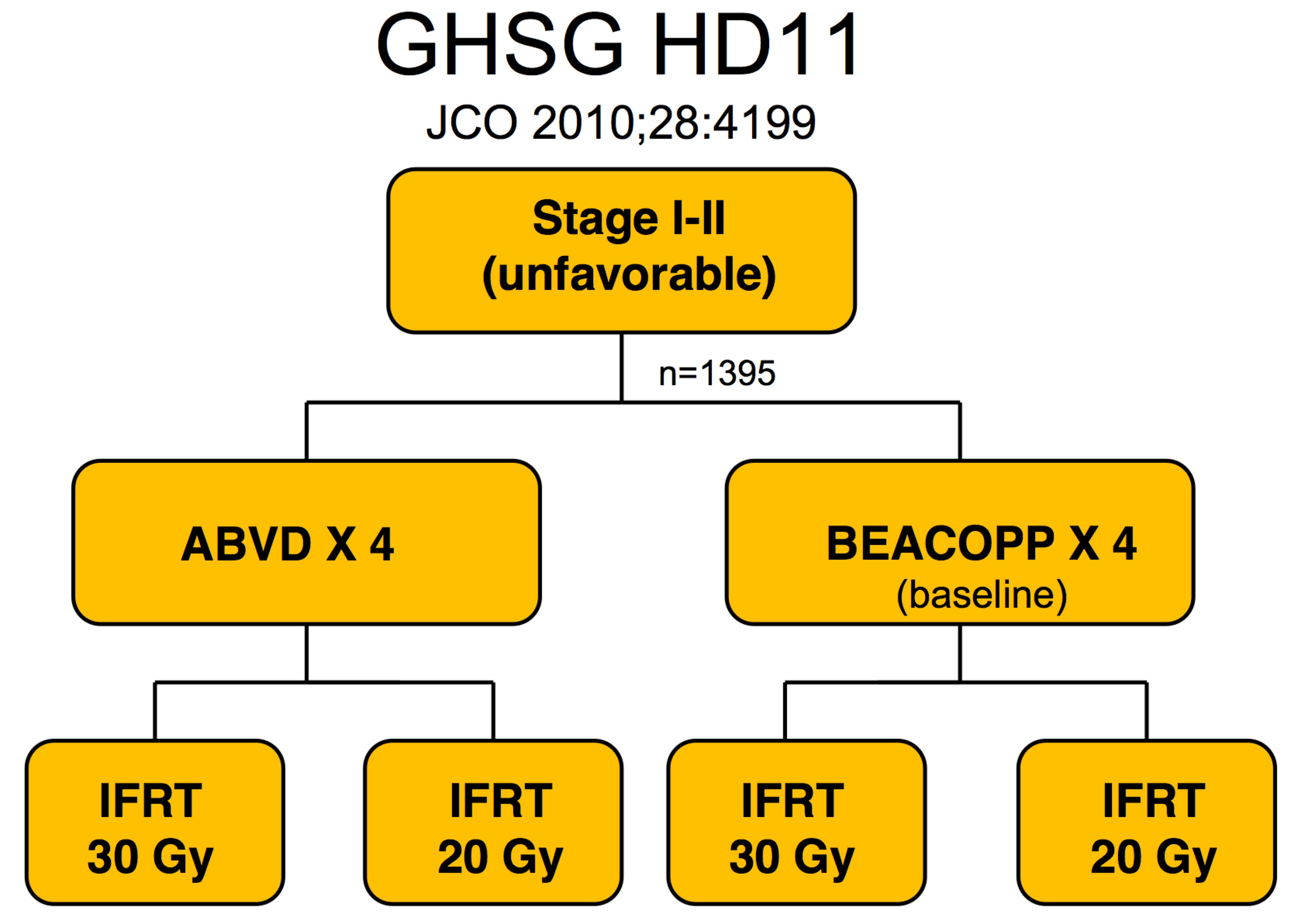
|
⇒ |
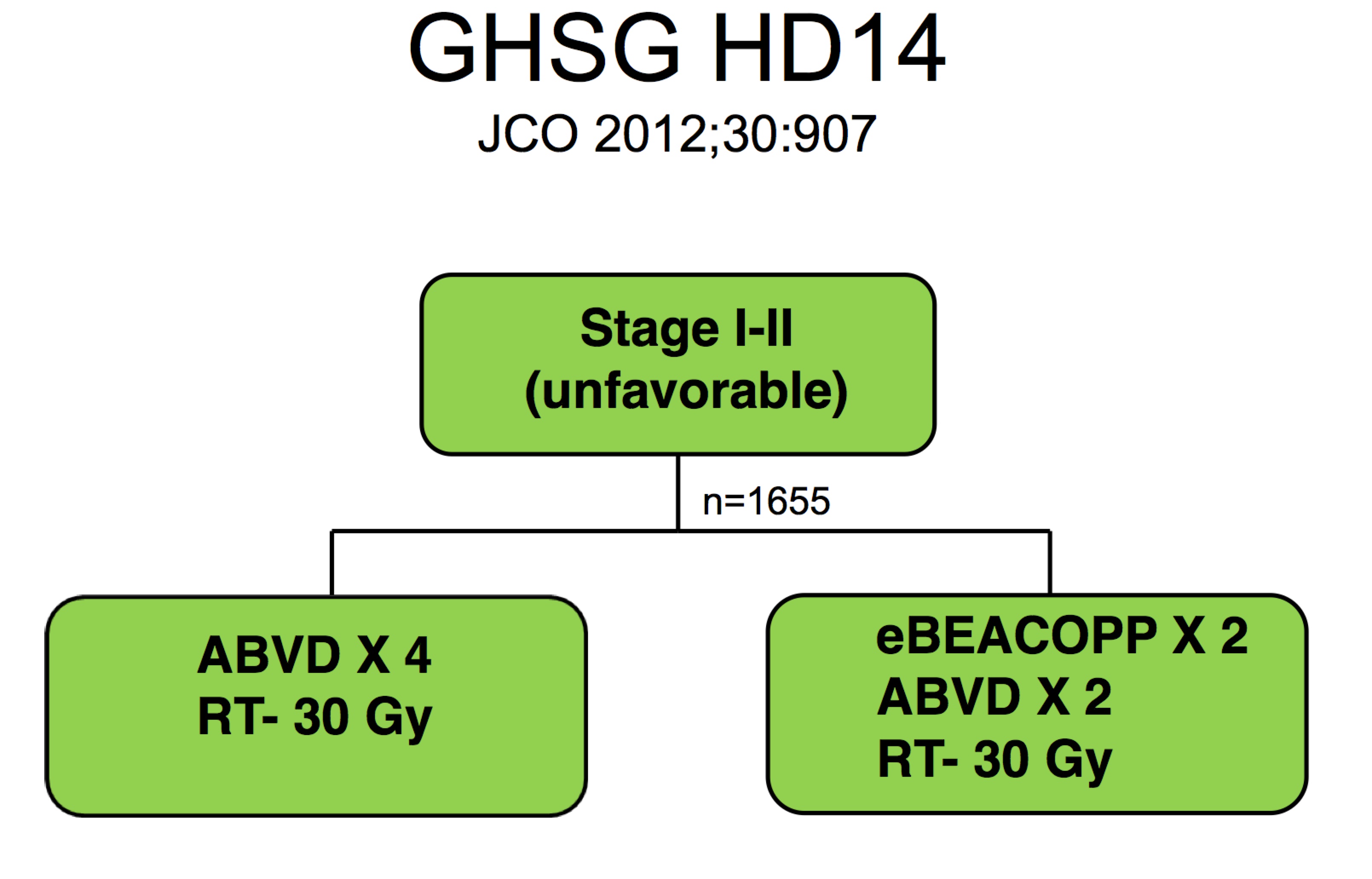
|
The follow on study (von Tresckow et al, Köln, & Würtzburg, JCO 2012;30:907) used "dose intensification." The study looked at BEACOPP x2 → ABVD x 2 → RT/ISRT to 30 Gy. The alternate arm was ABVD x 4 → RT/ISRT to 30 Gy. Both arms used 30 Gy and both arms used 4 cycles of chemotherapy. Based on this study, the standard for early unfavorable disease is 4 cycles of chemotherapy and 30 Gy of radiation.
For early unfavorable Hodgkin Lymphoma, treat with 4 cycles of chemotherapy followed by ISRT to 30 Gy .
Further Treatment Selection: NCCN and Deauville Critera (PET/CT)
The NCCN now incorporates Deauville PET criteria to assess response and bases subsequent treatment planning on the response to chemotherapy. The algorithm uses ABVD x4 cycles (except for "very favorable") followed by a repeat PET/CT. If Deauville Critera 0-3 are met (basically, is the disease more avid or less avid than the liver or mediastinal blood pool?), then proceed to ISRT. Otherwise, (ie doesn't meet response criteria), proceed to biopsy. If the biopsy is positive, then proceed to second line chemotherapy.
After second line chemotherapy, restaging using PET/CT and Deauville Criteria. If there is resolution, then proceed to high dose therapy and autologous stem cell rescue. ISRT may or may not be used. This is followed by brentuximab.
If there is persistently positive PET (Deauville 4), then stem cell or ISRT or additional chemotherapy may be considered followed by brentuximab.
If the biopsy is negative, proceed to ISRT.
Nodular Lymphocyte Predominant Hodgkin Lymphoma
For Nodular Lymphocyte Predominent HL (NLP-HL), the NCCN is presently recommending ISRT as a preferred treatment modality over observation in Stage I/II A, non-bulky disease. For bulky disease and "B" signs, chemotherapy ± retuximab are recommended followed by ISRT. For Stage III/IV-A/B, chemotherapy ± ISRT (sites of bulky disease).
Doses in advanced lymphoma are generally 30 Gy except for PET positive residual (Deauville 4-5), where higher doses of 30 Gy to 45 Gy are used. With bulky disease, occasionally 36 Gy is preferred, but 30 Gy is the most common dose.
Radiation Therapy Treatment Planning And Techniques
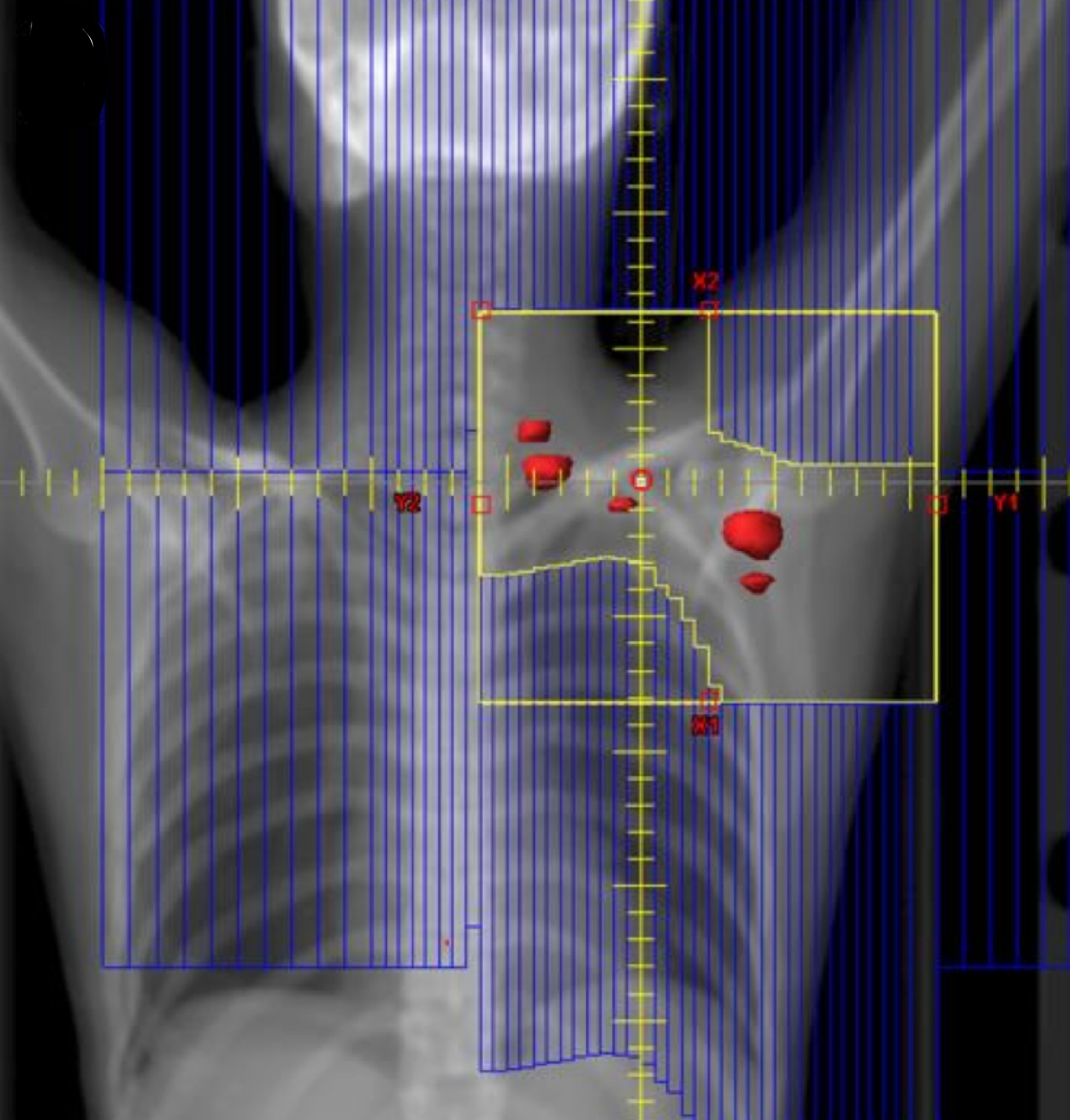
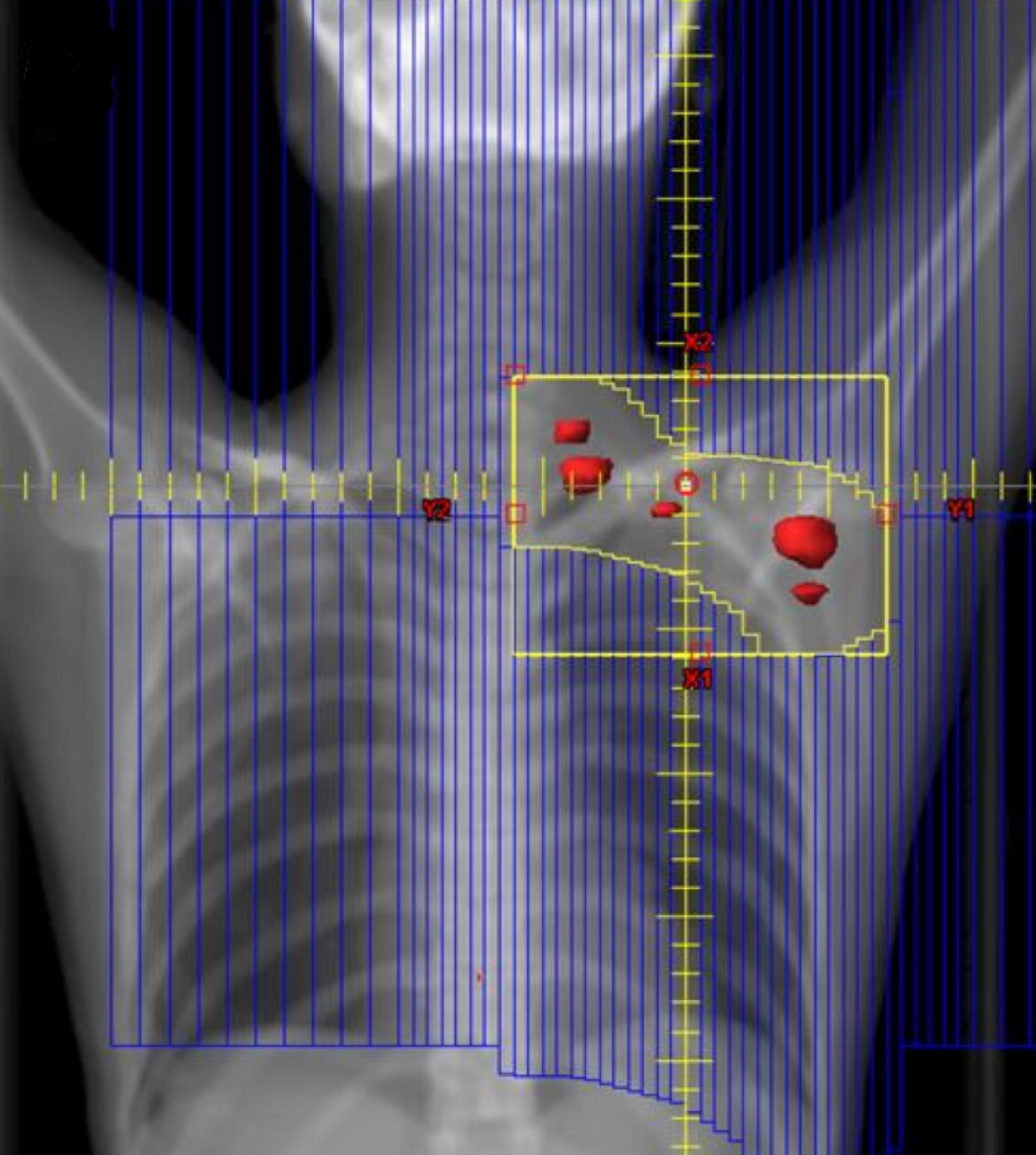
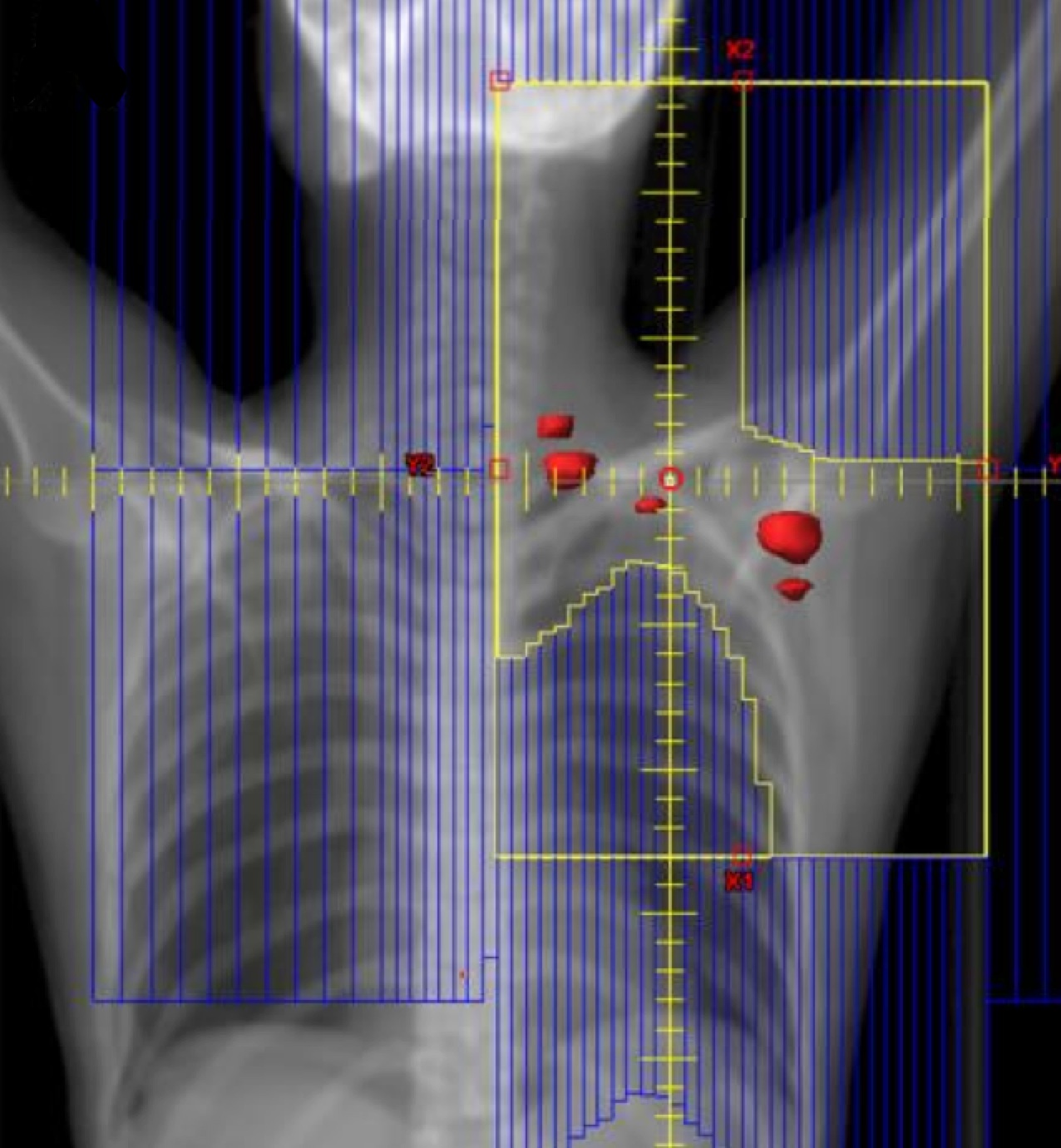
ISRT is most commonly recommended now as the most appropriate treatment field (Yaholom). Determining the optimum involved site definition may require incorporation of PET and MRI as well as pre-treatment and treatment planning CT to define the optimum volume.
 |
 |
 |
|
Involved Site Radiation Field
|
Involved Node Radiation Field
|
Involved Field Radiation Field
|
ISRT targets the site of the originally involved lymph nodes. This volume encompasses the pre-chemotherapy/pre-operative volumes. The fields are modified to spare adjacent uninvolved organs (lungs, bone, muscle, kidney), when the disease shrinks following chemotherapy. Concerns about the precise accuracy and localization of pre-treatment disease may lead to an expansion of the CTV based on clinical judgement. For NLPHL where radiotherapy is the sole modality treatment, a larger field should be considered.
Outcomes, Patterns of Failure, Prognostic Indicators
Follow up with an oncologist should continue for 5 years. Initial follow up is quarterly visits with H&P, then semi-annually in years 2-5, and annually thereafter. A complete response (CR) including PET should be documented within 3 months of treatment completion. Thereafter, an additional CT scan in the first 12 months, then as clinically indicated.
| Study | PFS5 yr | Treatment |
|---|---|---|
| GHSG HD10 | 91.6% | ABVD x 2 → 20 Gy |
| GHSG HD11 | 87.2% | ABVD x 4 → 30 Gy |
| GHSG HD14 | 95.4% | eBEACOPP/ABVDx4 → 30 Gy |