SBRT in Early Stage Non-small Cell Lung Cancer
Overview
Stereotactic Body Radiotherapy, also known as SABR or stereoablative radiation therapy is a means of delivering a very high dose per fraction in a few fractions with the intent to ablate tissue within the high dose region. Similar techniques have been used in brain irradiation for isolated disease in one or a few fractions. The present theory is that SABR/SBRT is that the radiobiology of radiation therapy changes once the dose/fraction is above a certain threshold, thought to be around 8 Gy, from a linear quadratic model to a cellular destructive model. Commonly, SBRT uses doses of beteen 10 Gy and 30 Gy per fraction in 5 to 3 fractions, delivered to a very small volume encompassing the lesion.
Studies have demonstrated poorer local control is associated with lower peripheral doses to the tumor volume. Therefore doses for SABR/SBRT should be prescribed to the lowest acceptable dose at the periphery rather than the isocenter dose. Local control appears to be a function of a BED10 ≥ 100 Gy.
Proximal Bronchial Tree Lesions — Increased Risk and Safety Issues
Early SBRT in lung cases demonstrated significantly higher risks, including mortality, if administered in very high dose/fraction to the proximal bronchial tree as described in RTOG 0236. Timmerman has reported increased mortality from high dose/fraction central tumors. Older studies using endobronchial brachytherapy treatments have likewise shown increased toxicity and mortality from treatment of central bronchial lesions.
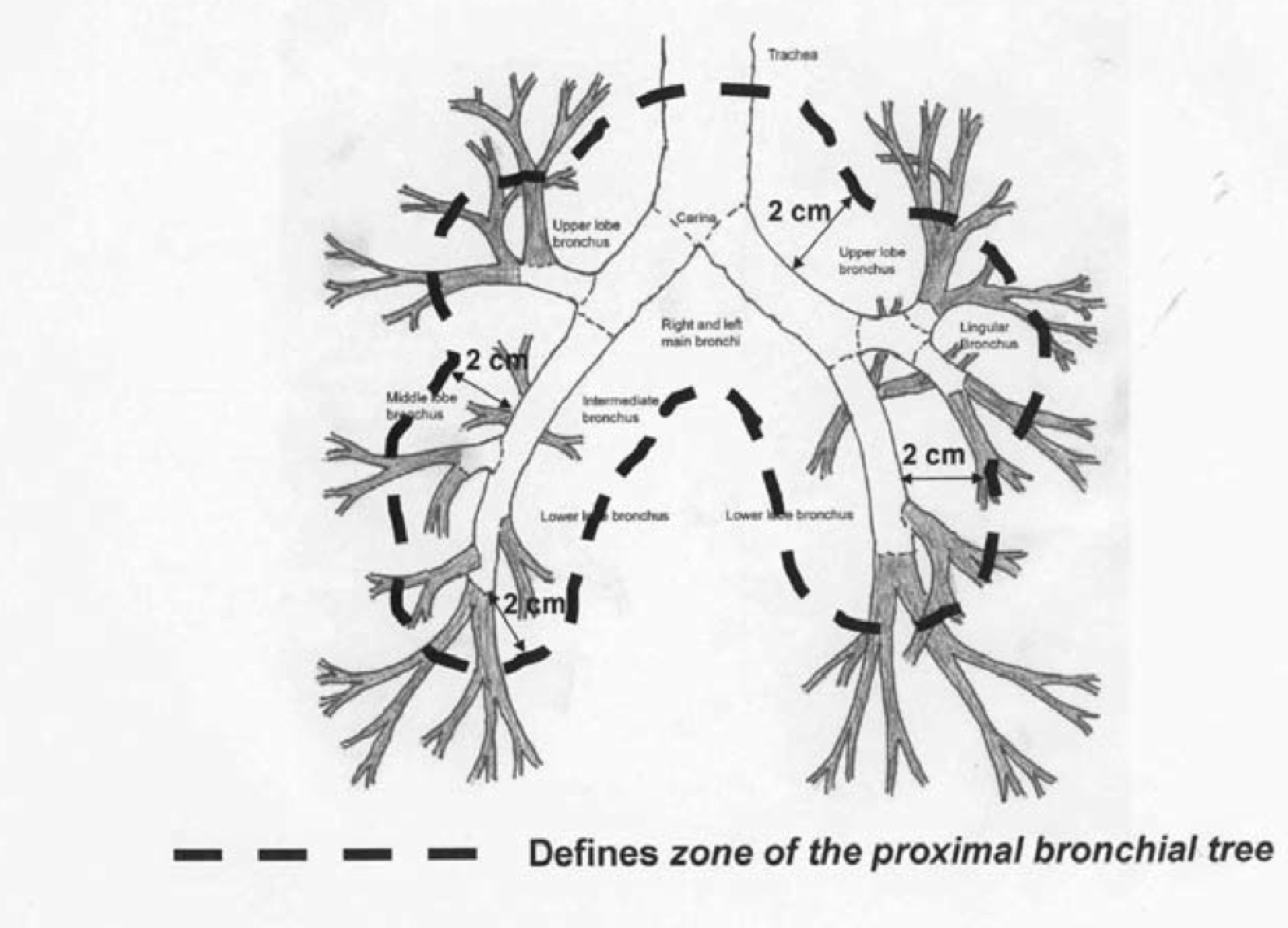
Several subsequent RTOG studies have excluded proximal disease cases from the studies. Slotman's group (NL) did a systematic review of studies for outcomes and toxicity of central tumors. (Senthi, S, et al, Outcomes of stereotactic ablative radiotherapy for central lung tumors: A systematic review, Radiotherapy and Oncology, 2013; 106:276-282) This paper notes that early SBRT studies using doses of 20-22 Gy/fraction to 60-66 Gy to the central region were associated with a 10-fold greater risk of high grade toxicity or death. RTOG 0813 examined central tumors to determine the maximum tolerated dose which can be delivered in 5 fractions. RTOG 0813 suggests that the risk associated with SBRT for central lesions may be prohibitive, however Dutch and Japanese investigators have reported favorable outcomes at 6-7 Gy/fraction to 48-60 Gy.
The VU study found that there was no difference in median survival at 24 months between central and peripheral lesions. OS3 was similar at 64% v. 51% (p=0.09). It thus appears that central v. peripheral location does not impact survival.
When toxicity is reviewed, the VU/Amsterdam review found that a peripheral BED10 ≥ 100 Gy was important for local control and a BED3 ≤ 210 Gy decreased the risk of mortality to under 1%.
Since pneumonectomy is the alternative for central tumors, this compares favorably to peri-operative mortality rate of 4.5% and surgical complication rates of 25%, with an 8% risk of bronchial stump complication (bronchial necrosis, wound dehiscience, stricture and fistula formation).
Peripheral lesions risk includes injury to the chest wall, and in particular to ribs.
Clinical Workup and Evaluation
General Management and Treatment
Stage IA non-small cell lung cancer
Radiation Therapy Treatment Planning And Techniques
Dose Specifications for SBRT
SBRT has been formally defined by the ACR and ASTRO. The term SBRT encompasses the targetting, planning, and directing of therapy using external beams along any trajectory in 3D space toward a target of known 3-D coordinates. In addition, the definition should include a volume surrounding a known point in space. I find it easier to think of this as volume defined by spherical coordinates with origin at R (cartesian: x,y,z,t) a distance r from the center of the origin, encompassing θ = 0 to 2 π , φ =0 to 2π and a "wobble" describing the change in position over time of the respiratory cycle, t=0 to resp, where resp is the mean time of respiration calculated to define the precise tumor motion over the respiratory cycle time.
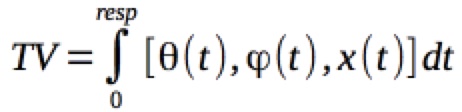
Once a target coordinate/volume is identified, this must be related to the fixed external coordinate system which can then be used to establish beam portals. Traditionally, this coordinate reference frame has used the boney landmarks of the body with localization marks provided by permanent tattoos using laser fiducials in anatomically stable locations. In SBRT, the instability of the skin marks and bony landmarks with respect to the tumor's physical location in space is inadquate. We must therefore directly image the desired treatment location. More recently, this has been done with Tomotherapy and/or Conebeam CT localization of the tumor and with 4DCT based treatment planning.
Simulation and Localization
Patients are immobilized in the treatment position, and to the greatest extent posslbe, with arms overhead. Some patients are unable to fully extend their arms, but the better we can clear the torso, the more options are available to provide satisfactory beam angles to treat the disease.
Positioning must be in a stable position with a full length vac-loc. Patients should be comfortable in the treatment position to avoid errors in reproducibility due to discomfort during treatment. Respiratory motion can be reduced by using a bag with mild vacuum applied (Body-Fix) system of vacuum bagging. In this setting a plastic bag is placed over patient's torso with head out of the bag, and the bag sealed to the vacloc with mastic to prevent air leaks and a mild vacuum is applied. Once this is completed and leak sealed, respiratory motions are captured either with camera techniques or other reliable respiratory motion analyzers. Patients should be coached as to proper breathing prior to simulation scan acquisition, and the scanner should be started but no imaging done to avoid startling the patient. Once the patient is comfortable, stable respiration patterns are observed, then cine images with respiratory binning should be obtained with 2.5 mm cuts.
Technical Factors and Treatment Planning
Treatments are generally delivered with 6 MV photons. Higher energy photons should be avoided when the beam passes through significant distances of lung. The loss of transient charged particle equilibrium may reduce dose to the incident side of the tumor, causing a potential loss of dose in the secondary buildup region on the leading edge of the tumor. Small fields also contain dosimetric uncertainty. For these reasons, field sizes on conventional accelerators without specialized collimation should be restricted to 3 cm2 equivelant square or larger for 3-D conformal fields.
IMRT is used and was allowed as part of the RTOG 1021 protocol comparing wedge resection to SBRT. Caution should be used to insure that there are no significant dosimetric errors, particularly those associated with tumor motion. The risk of the tumor motion carrying the tumor out of the field at the same time the IMRT dose attenuation takes place may result in significant underdosing. Care should be taken to insure that the leaves are not covering the tumor and reducing dose to the tumor during tumor excursions during the respiratory cycle. The safest way to insure this is to limit the use of IMRT to those cases where tumor motion is less than 5 mm.
The Target Volume
SBRT uses the gross (or visualizable) target volume of disease. This is expanded or altered for control and correction of respiratory motion. Generally respiratory/organ motion is captured using a mechanism which tracks respiratory activity. There are a number of different systems in use, including electronic (ie tocometer -- Seimens, camera motion tracking -- Varian) and others. Cruder techniques include capturing a series of images with free breathing, merged/fused with various breath hold images to attempt to track respiratory motion. Most have adopted an electronic means of 4d ct acquisition and binning of images. These are then used to guide the construction of an intermediate ITV target volume, which incorporates target motion and deformation throughout the respiratory cycle. From this a CTV/PTV can be constructed.
Although a recommendation for contrast enhanced 4d-CT has been made, technical and institutional limitations may preclude this. Also, patient contrast burdens are a factor when contrast enhanced CTs are repeated in close proximity. Therefore, if a prior contrast enhanced CT is available, and if good image fusion can be achieved, it may be possible to forego contrast enhancement on the respiratory gated 4d-CT and use the non-gated diagnostic CT to provide contrast enhanced information for treatment planning purposes. RTOG 0915 permits this to reduce the contrast burden, provided the diagnostic image was obtained no earlier than 8 weeks prior to SBRT.
- Target Volume
- The visualizable disease with CT imaging set to lung windows. The acquisition CT should be done with IV contrast, if possible to permit visualization and discrimination of vessels and atelectasis. CT images should be 2.5 mm cuts. The GTV should be precisely conformed to the visualized disease without expansion. Some have proposed identifying the tumor on a maximum intensity projection scan reconstruction from the binned images. We have found that doing so may underestimate outer excursion limits and recommend drawing the target using lung windows on each phase scan of the cycle. We construct the ITV from each of the individual GTVs on each respiratory binned set. There is no CTV expansion for microscopic disease. GTV/ITV=CTV.
- ITV
- The ITV is constructed by collecting the total volumes visualized in constructing the target volumes on the individual 4D respiratory gated series. The ITV is essentially a Boolian OR function where all the areas defined on visualization are included and formed into an ITV.
- PTV
- The PTV expansion is 5 mm axially, and 1 cm superiorly and inferiorly on this protocol. This expansion is used when non-4D respiratory gated imaging is used to construct the GTV/CTV. RTOG-0915 provides a second method of constructing the PTV, is a 5 mm uniform expansion around the GTV/ITV, when 4d gating is used.
Dosimetry
Prescription lines covering the PTV will generally be the 60-90% isodose volume. Hot spots (IDV > 100%) should be placed within the ITV/GTV, and not allowed in adjacent tissue.
Various definitions, some good some bad, some descriptive (or not so descriptive) have been used. This terminology is a mess, but we may be stuck with it. Following is a glossary of the terms being used today.
- Maximum Dose
- Prescription Isodose Volume
- The prescription isodose surface must be ≥ 60% and < 90 of the maximum dose. The PTV should be covere by 95% of the prescription isodose, and 99% of the PTV should receive a minimum of 90% of the 90% isodose volume.
- High Dose "Spillage"
- This is a funky term that describes hot spots which is defined as all tissue outside of the PTV which receives > 105% of the prescription dose. Another was of discribing this is V105%. The RTOG protocol limits this to no more than 15% of the PTV volume. (This should be described in the OAR limitation section). The main intent is to constrain the spatial location and dose gradient parameters of hot spots. See conformality ratio index below.
- Intermediate Dose "Spillage"
- Another funky term which is the fall off gradient beyond the PTV is described. Intermediate dose spillage criteria for location is Maximum total dose to any point ≥ 2 cm from the PTV.
In other words, the ratio of the volume of the 50% IDV to the volume of the PTV ≤ CI(PTV_volume), where PTV_volume value is a function of the PTV volume.
- Conformity Index
- The Conformity index is a ratio of volumes:

Generally the desired CI is between 1.2 and 2.0
Organs At Risk Dose limits used with RTOG 0915
| Organ | Constraint |
|---|---|
| Spinal Cord | Dmax < 6.5 Gy/fraction (26 Gy) |
| Lung | 1500 cm3 ≤ 2.9 Gy/fraction (11.6 Gy) |
| Lung | 1000 cm3 ≤ 3.1 Gy/fraction |
| Esophagus | ≤ 4.7 Gy/fraction, 30 Gy maximum |
| Heart/Pericardium | < 15 cm3 < 7 Gy/fraction |
Organs at Risk Dose limits described by the NCCN based on RTOG data
| Organ | Constraint | ||
|---|---|---|---|
| Single Fraction | 3 Fractions | 5 Fractions | |
| Spinal Cord | 14 Gy | 18 Gy (6 Gy/fx) | 30 Gy (6 Gy/fx) |
| Esophagus | 15.4 Gy | 27 Gy (9 Gy/fx) | 105% of PTV prescription (central tumors) |
| Brachial Plexus | 17.5 Gy | 24 Gy (8 Gy/fx) | 32 Gy (6.4 Gy/fx) |
| Heart/Pericardium | 22 Gy | 30 Gy (10 Gy/fx) | 105% of prescription PTV (central tumors) |
| Great Vessels | 37 Gy | Not Specified | 105% of PTV prescription (central tumors) |
| Skin | 26 Gy | 24 Gy (8Gy/frx) | 32 Gy (6.2 Gy/fx) |
Outcomes, Patterns of Failure, Prognostic Indicators
The Japanese experience reported in 2001 used SBRT 5 - 10 fractions for 1 - 2 weeks for a total of 50 Gy - 60 Gy. At 36 months, local progression was not seen in 94%, and OS3 was 66% in 50 patients and 86% in the medically operable (but refused surgery) patients. CSS3 was 88%. Adverse events were minor bone fracture and temporary pleural pain.
Side Effects and Complications of Treatment
Radiation toxicities of SBRT are a function of peripheral v. central location, dose/fraction, concentration of radiation in sensitive organs, and overall dose. Timmerman (UI) looked at Stage IA/B NSLCL with dose escalations starting at 8 Gy x 3 (24 Gy). These studies noted that patients receiving > 16 Gy / fraction had signficantly improved outcomes, and a maximum tolerated dose appeared to be 72 Gy. Dose limiting toxicities were bronchitis/bronchial necrosis, pericardial effusion, hypoxia, and pneumonitis.
Timmerman did a follow on study which treated 70 patients to 60 Gy to 66 Gy in 3 fractions over 1 to 2 weeks. He found a major response rate at 60% in 3 months post treatment. Grade 3 - 5 toxicity was found in 14/70 patients, with 6 deaths. The median time to observation of side effects/toxicity was 10.5 months.Tumors with GTV > 10 ml had an 8-fold risk of developing high grade toxicity. Central lesions had a much worse toxicity profile with 46% severe toxicities compared with peripheral tumors at 16%