CNS Primary Tumors - Generally
Anatomy
Epidemiology
Pathology
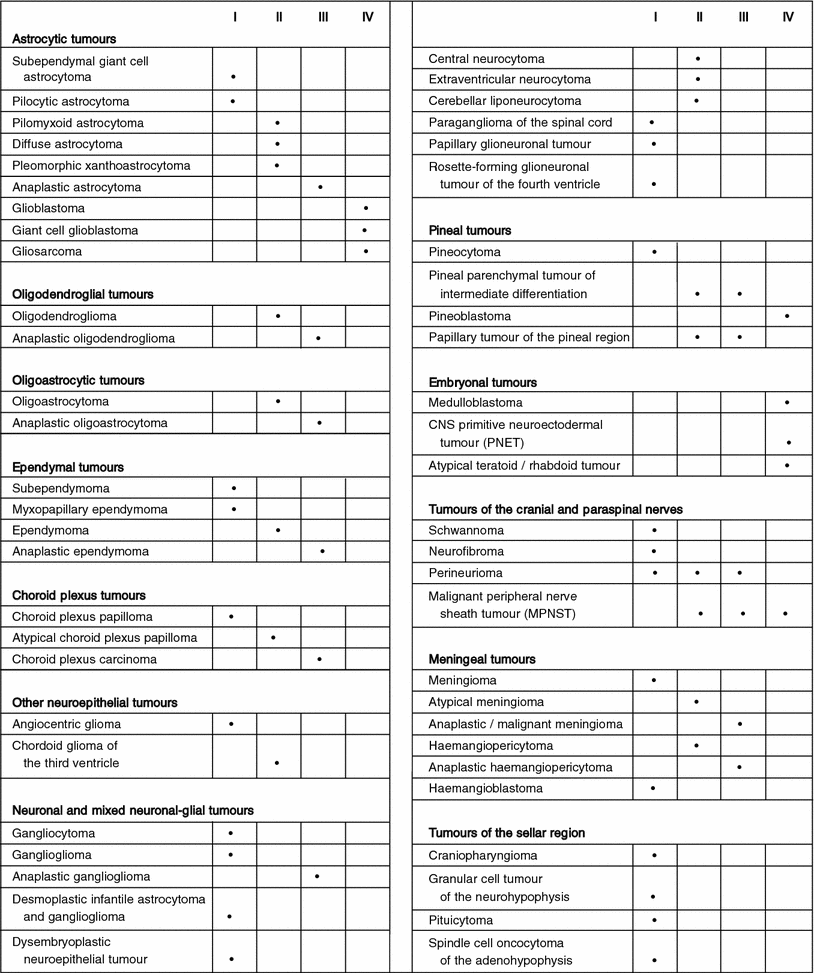
Intracranial tumors are of ectodermal and mesodermal origin and arise from the brain, cranial nerves meinges, pituitary, pineal and vascular elements. The present classification systems is the WHO grading and lists more than 100 categories of CNS malignancies.
The following table shows some of the brain tumors categoried by their histological types.
| Histological Type | |
|---|---|
| Astrocytic | Pilocytic (juvenile) astrocytoma |
| Well-diff. low grade astrocytoma | |
| Anaplastic astrocytoma | |
| Glioblastoma multiforme | |
| Ependymoma | |
| Choroid plexus papilloma | |
| Oligodendroglioma | |
| Mixed gliomas | |
| Medulloblastoma | |
| Anaplastic anglioglioma | |
| Pineal parenchymal tumor of intermediate diff. | |
| Histological Type | |
|---|---|
| Craniopharyngioma | |
| Meningioma | |
| Schwannoma | |
| Lymphoma |
WHO Grading and Tumor Classification 2
Natural History
Natural history of CNS tumors is determined by location, grade, and histology. Gliomas tend to infiltrate without forming a capsule. They frequently cause vasogenic edema in surrounding tissue, but can also cause ischemic edema. Edema is well imaged on T2 weighted MRI and is usually responsible for at least some of the clinical signs and symptoms. Edema results from altered blood brain barrier. Tumors cause varying amounts of edema:
- Metastasis (most edema)
- astrocytomas
- meningiomas
- oligodendrogliomas (least edema)
High grade tumors can metastasize by seeding the subarachnoid and ventricular spaces. Those tumors will follow the flow of CSF and cause "drop metastases" along the cord or in the caudal portion of the spinal canal. Tumors that spread along the CNS include:
- medulloblastomas
- primitive neuroectodermal tumors (PNET)
- CNS lymphomas
Other tumors that may spread along CSF pathways might include germ cell tumors and ependymomas. Rarely, extra-cranial metastases from medulloblastomas germinomoas and high grade astrocytomas are found.
Clinical Workup and Evaluation
Presentation
CNS tumors may present as generalized or focal. Headache is more prevalent in faster growing high grade tumors. Seizures are more common in lower grade, slower growing tumors. Focal neurologic deficits (focal weakness, language dysfunction, sensory loss) are more common in high grade tumors.
Workup
Initial work up of brain tumors includes a complete history and physical examination. Information from relatives is useful, as there may be changes in mentation and recall associated with the primary tumor. Biopsy confirmation is helpful, but certain sites are not amenable to biopsy (brainstem). If there is evidence of systemic spread, buipsy confirmation of the primary tumor is recommended. If there is an extracranial primary (lung, breast, colon, melanomas), these are far more likely to be a cerebral metastases than a CNS primary.
Imaging Studies
MRI with gadolinium is the sine qua non of CNS tumor identification. CT is reserved for those for whom MRI is contra-indicated, including implanted pacers, metal fragments in the eyes, paramagnetic surgical clips. Imaging techniques for brain tumors include the following series:
- T1 high resolution sagittal images wo/with gadolinium
- T1 axial images wo/with gadolinium (architecture)
- T2 and FLAIR axial sequences (edema)
Post treatment imaging including MR-Spectroscopy may enable discrimination of pseudo-progression of tumor due to treatment effect (radionecrosis) and avoid a surgery. F-DOPA PET has not yet been approved in the US but is used and being evaluated in Europe.
For tumors with the increased likelihood of CNS dissemination, the neuroaxis must be imaged for complete staging. This should be performed prior to surgery or delayed to > 3 weeks post-operative to avoid confusing bleeding and operative changes being confused for malignancy. An increased dose of gadolinium may be useful and sensitive to find leptomeningeal spread in the post operative setting.
Surgical Confirmation and Biopsy
Surgical staging is improving with the advent of STEALTH and stereotactic biopsy techniques the safety and yield has improved. For patients with known extra-cranial primary tumors, and multiple lesions consistent with metastatic disease, it is reasonably safe to assume that the findings are malignant and recommend treatment accordingly.
There are some areas of the brain it is not safe to biopsy. These may also be diagnosed based on imaging findigs. Such sites include the brainstem, and optic nerve.
CSF cytology is essential for staging tumors with CSF dissemination propensity such as medulloblastoma, PNET, germ cell tumors, and CNS lymphomas. Sampling the CSF in an immediate post-operative period may lead to false positives, and should be performed before surgery or delayed 3 weeks or more post-operatively. Pre-operative spinal taps should not be performed if there is evidence of elevated intra-cranial pressure. CSF findings in the presence of metastases may include:
- Elevated CSF pressure > 150 mm H2O
- Elevated Protein > 40 mg/dL in the lumbar cistern
- Decreased glucose levels < 50 mg/ml
- CSF cytology positive for malignancy
- Tumor markers in CSF may be important in confirming a diagnosis
Non-neoplastic Causes of Imaging Findings
Prior to treating a CNS disease, it is important to consider other causes which may be non-malignant. The differential diagnosis of CNS imaging findings for space occupying lesions is:
- Neoplasm —Primary
- Solitary with no prior history of cancer, contrast enhancing, thick and/or nodular
- Neoplasm —Metastatic
- Multiple lesions, prior cancers, edema present, located at gray/white matter junctions
- Infections — Abscess
- Fever, acutely ill ± systemic infection, cyst cavity with smooth thin walls, Contrast enhancing, restricted diffusion within the cavity.
- Infections — Cerebritis
- Fever, acutely ill, ± systemic infiection, diffuse T2 change, no contrast enhancing mass
- Infections — Menengitis
- Diffuse enhancement of the meninges on T1 imaging (which may simulate leptomeningeal metastases)
- Vascular — Infarct
- Gray and white matter involvement, wedge like vascular distribution associated with restricted diffusion
- Vascular — Bleed
- Homogenous, clears quickly with residual hemosiderin ring
- Treatment Related Necrosis
- Central hypodensity, contrast enhancing ring, edema > 6 months post radiaiton or chemotherapy, metabolic scan shows low activity.
General Management and Treatment
General management of patients with brain tumors include management of symptoms until definitive treatment can be arranged. This includes the management of increased intracranial pressure, seizures and venous thromboembolic disease.
Edema
Glucocorticoid steroids are used to control edema, and thus neurologic signs and symptoms caused by cerebral edema. Lower doses of dexamethasone (2 mg - 4 mg) BID are as effective as higher doses. Steroid use is not benign and steriods should be tapered as quickly as possible. It may be impossible to completely taper steroids. In these cases, as low as dose as possible which provides symptom relief is the goal. Slow tapers are necessary to allow physiologic steroid recovery and to prevent rebound edema.
Seizures
Patients with seizures require anti-convulsant management. Keppra is the most common anti-convulsant in use today, but diphenylhydantoin, carbamazepine, phenobarbital have all been used. The latter drugs all induce P450 which increase the metabolism of anti-neoplastic drugs, and thus are not preferred agents when chemotherapy is planned.
Prophylactic use of anti-convulsants in patients who have not had seizures is controversial. The NCCN and American Academy of Neurology do not recommend using prophylactic anti-convulsant therapy, due to a lack of data.
Surgery
Surgical procedures are a.) biopsy for diagnosis only, b.) resection for cure, c.) surgical debulking for management of mass effect symptoms, d.) re-resection to distinguish recurrence v. radio-necrosis v. pseudo-progression. Complete resection is associated with a survival advantage for high grade gliomas. For others such as primary CNS lymphoma, chemotherapy and radiation without surgery make aggressive resection unnecessary and the role of surgery is to provide tissue for diagnosis.
Radiation Therapy Treatment Planning And Techniques
Radiation injury to the brain is a poorly understood process which is highly complex. It is a function of several factors including dose, treatment volume, dose/fraction, and specific target cells. Secondary mechanisms of injury are also possible, with vascular leaks causing edema, vascular endothelial loss causing hypoxia, reactive gliosis and host factors. Certain structures are significantly more radiosensitive, including:
- lacrimal gland (25 Gy)
- Optic Chiasm (50 Gy)
- hypothalamus (54 Gy)
- lenses
The time course for manifestation of radiation injuries is highly variable and easily confused with tumor progression.
Outcomes, Patterns of Failure, Prognostic Indicators
Side Effects and Complications of Treatment
Radiotherapy acute toxicities include transient worsening of pre-treatment deficits, with further acute toxicities up to 6 weeks post completion of treatment. These are generally felt to be the result of transient peri-tumoral edema and respond to a brief increase of steroids. Persistent or refractory symptoms may be the result of tumor progression and repeat imaging while on treatment may be indicated if clinical conditions worsen despite steroid treatment or increase.
Constitutional symptoms (fatigue, headache, drowsiness) are seen sometimes in patients treated with whole brain or cranio-spinal irradiation. Alopecia is nearly universal and may be permanent with higher doses. Nausea and vomiting may occur, particularly with posterior fossa irradiation.
Subacute toxicity develops in the 6 week to 6 month time frame and is attributed to capillary permeability changes and transient demylination due to injury to oligodendroglial cells. This include somnolence, fatigue, and deterioration of existing deficits. The phenomenon of pseudo-progression is consistent with this time frame and complicates medical decisions as it may not be readily determined from imaging if there is pseudoprogression or recurrence of disease.
Late sequale of radiation appears between 6 months and years. Late effects are usually irreversable and progressive. The most serious late radiation injury is radio-necrosis which peaks at 3 years. Radiation necrosis can mimic recurrent tumor, The best treatment for radio-necrosis is control with steroids followed by surgical debulking which will also provide a tissue diagnosis. Necrosis after debulking may still progress.