Pediatric Rhabdomyosarcoma
Epidemiology
Rhabdomyosarcomas are tumors that originate from embyonal mmesenchymal (skeletal muscle) cell lines. They can occur anywhere in the body, are locally invasive and rapidly disseminate. The prognosis is location, histopathologic and TNM dependent, although AJCC staging systems are not commonly used. They are most frequently found in the following sites:
- 9% orbit (favorable site)
- 7% head and neck — non-parameningeal (favaroble site)
- 13% trunk
- 7% retroperitoneum
- 31% genitourinary &msdash; (non-bladder,non-prostate) (favorable)
- bladder or prostate (unfavorable site)
- 25% parameningeal (unfavorable site)
- 13% extremity (unfavorable site)
- 3% other sites (unfavorable site)
RMS is the most common childhood soft tissue sarcoma with an incidence of 4.4/million (white), 1.3/million (black). The majority are under 10 years old, and 5% under a year. There are two age peaks: 2-6 and adolescence. Younger patients tend to have embryonal histology. Older patients tend to have alveolar histologies. Age is a prognostic indicator: < 1 and age > 10 have worse survival. Adults also have poor outcomes, but when treated with aggressive pediatric regimens, they do better, and the prognosis may be similar.
Natural History
The precise cause is unknown, but may be associated with disorders in development, including CNS, GU, GI or cardiac abnormalities, plus the usual suspects. Gorlin nevoid basal cell syndrome, congenital pulmonary cysts, neurofibromatosis, Li-Fraumeni, Beckwith-Wiedemann, Costello syndromes.
Tumors arising in the bladder and vagina occur primarily in infants. These tumors are frequently embryonal or botryoid type tumors.
Tumors of the trunk and extremity occur in adolescents and are often alveolar or undifferentiated types.
Tumors of the head and neck are commonly embryonal (do better) and occur throughout childhood.
Paramenengeal sites are a subdividision of head and neck sites: ,, middle ear and mastoid space, infratemporal fossa and pterygopalatine and parapharyngeal sites.
- nasal cavity
- nasopharynx
- paranasal sinuses
- middle ear
- mastoid space
- infratemporal fossa
- pterygopalatine space
- parapharyngeal space
Rhabdomyosarcomas are locally invasive tumors which frequently form a pseudocapsule. They have the potential to spread long fascial and muscle planes, do extend into the lymphatics, and have hematogneous dissemination. The risk for lymphatic spread is 15%, but varies with the site of lesion. Lymph node mets are rare in orbital tumors, but most commonly occur in tumors of the nasopharynx and metastasize to nodes in about 15% of head and neck presentations. Regional lymph extension is seen in 25% of paratesticular, extremity, and truncal tumors. Hematogenous spread is seen in 15%, particularly those with truncal or extremety primaries. The most common sites of distant metastases are the lungs, bone marrow and bone. Malignant pleural and peritoneal effusions are seen in chest, abdominal, and pelvic sites.
RMS results have improved substantially over time. Early RMS treatments were reported based on surgical "staging" systems that were more descriptive of the initial surgery chose, the surgical pathology and surgical outcome. More recent studies are based on TNM approaches to treatment. Progressive improvements in survival have been demonstrated by IRS 2-3 between 1978 and 1991. IRS-IV/V changed philosophy from the surgical based predecessors to a more biologically based system between 1991 and 2006. The COG (=POG+CCG) and SIOP (EU) and CSTG (UK) are now contributing to rhabdomyosarcoma treatment advances.
- IRS 1 (1970s) OS5=55%
- IRS 2 (1982s) OS5=63%
- IRS 3 (1988s) OS5=71%
- IRS 4 (1990s) OS3=86% (non-mets) 39% (mets)
- IRS 5 (2000s)
Pathology
Rhabdomyosarcomas are divided into favorable, intermediate and unfavorable risk groups.
Favorable: Embryonal, Botryoid, Spindle Cell
Embryonal is the most common type, accounting for 60-70% in childhood and arises in the head and neck or in the genitourinary tract. These cells arise from mesenchymal cells that tend to differentiate into skeletal muscle. There is a great variety of degree of differentiation, moderate cellularity and loos stroma in most cases. The cells are fusiform or stellate with cross-triations present in about 1/3. PAS stains are used, with actin/sesmin reactivity and Z-bands present. LOH 11p15.5 may be present. Differential includes all small round blue cells.
Botryoid is present in about 10% of RMS and occurs in mucosa lined organs including the bladder, vagina, nasophayrnx, nasal cavity, middle ear and biliary tree. It is loose, myxoid with a condensed tumor cell layer identifiable. Botryoid tumors are usually not invasive and are localized RMS.
Spindle cell variants are composed of spindle shaped cells with low cellularity most commonly found in the paratesticular region.
Unfavorable: Alveolar
20% have alveolar variants which most commonly are found in adolescents in trunk, extremeties, peri-anal and perineal regions. The alveolar form resembles developing skeletal muscle in the 10-20 week GA fetus.
Staging
Staging is a mess right now, but hopefully order is coming soon. Historically, multiple staging systems have been developed at multiple institutions and across multiple groups. These staging systems are finally evolving into two relatively broad categories based on intergroup (NA) and SIOP (EU) work. IRS III moved to a pre-treatment TNM staging system based on SIOP's system. This staging system incorporates size, invasiveness into the T-stage nodal status and metastases.
- Stage 1: favorable sites
- Stage 2: unfavorable sites small disease (≤ 5 cm)
- Stage 3: unfavorable sites large disease (> 5 cm) or N+
- Stage 4: any site with hematogenous metastases
The T stage is based on confinement to site of anatomic origin (T1), or with extensions beyond originating site (T2). The T-suffix is based on size (a ≤ 5 cm, b > 5 cm). Nodes are either positive or not, as is metatastases.
Note: for parameningeal sites workup include both BM biopsy and CSF cytology.
Clinical Group continues to have relevance as a reflection on the extent of surgery.
| Group I | Localized disease, completely resected a. Confined to muscle or organ of origin b. Infiltration outside the muscle or organ of origin |
| Group II | Total gross resection with
a. Microscopic residual disease b. Regional lymphatic spread, resected c. Both |
| Group III | Incomplete resection with gross residual disease
a. After biopsy only b. After major resection (more than 50%) |
| Group IV | Distant metastatic disease present at onset |
The primary site is a strong determinant of outcome with favorable and unfavorable sites. This is incorporated in the IRSG stagign Groups. All favorable sites are Stage I. The primary reason favorable v. unfavorable sites are the amenability to resection, and thus the group placement.
Clinical Workup and Evaluation
RMS can develop in many primary sites. Clinical signs and symptoms are site specific. Generally it presents as an asymptomatic mass. Any presenting symptoms are attributable to mass effect on associated organs and tissue. Tumors of the orbit may cause proptosis and opthalmoplegia. Parameningeal tumors present with nasal, aural or sinus obstruction, CN palsy, and headache. GU tumors can present with hematuria, obstruction or constipation.
Diagnostic workup includes an expeditious local and systemic workup. These tumors grow rapidly. An initial assessment by all members of the interdisciplinary team permits accurate staging and the formation of a uniform treatment plan.
A history and physical exam focusing on determining the extent of local disease and presence of metastates. Local infiltration signs/symptoms should be clinically assessed. Tumors should be imaged with CT and MRI and plain films, depending on the site. GU can be imaged with ultrasound, voiding csytourethrogram, cystoscopy or pelvic EUA. Draining lymphatics are imaged using CT and also surgically staged.
The most common sites of mets are the lung, bone, bone marrow and local-regional lymph nodes Chest CT is the optimal imaging method for lung mets, a bone scan is used for bony mets, but is not reliable for Base of Skull invovlement with parameningeal tumors. For Parameningeal tumors, CT or MRI are used in the workup.
If there are parameningeal sites involved, then CSF should be sampled. MRI of the spine is included if there are cord-related symptoms. PET/CT is effective in identifying regional and distant metastases.
General Management and Treatment
The current COG protocols assign RMS patients into three distinct risk groups for the purpose of management. These are divided by histology, stage and surgical/clinical group.
- Low Risk: localized, favorable site with embryonal histology
completely resected embryonal disease at unfavorable sites (Group I) or only microscopic residual disease (Group II) - Intermediate Risk:Patient wiht embryonal RMS at unfavorable sites with gross residual disease (Group III) and patients with non-metastatic alveolar RMS at any site
- High Risk: Patients with metastatic RMS (Group IV or Stage IV)
The treatment management decisions are compounded by a goal of eradicating disease while preserving function and cosmesis. Aggressive surgery and radiation cures less than 25% except for orbital and GU primary sites. Conversely, chemotherapy alone demonstrates high local failure rates. Judicious use of chemotherapy to shrink local disease and eliminate distant mets and radiation to improve local control has lead to decreases in aggressive surgery. Current efforts are now underway to optimize these strategies.
Surgery
Complete resection was the original clear goal of RMS. Radical surgery such as pelvic exenteration, radical prostatectomy, cystectomy, amputations and orbital exenteration, but fewer than 10% were amenable to complete resection and cure. Most of the children undergoing radical surgery had severely compromised quality of life. Select sites were more curable with surgery alone than others: bladder, and orbit. The achievement of local control with organ preservation is now the surgical goal. To acheive this goal, combined modality therapy is required. Aggressive surgery remains paramount for salvage therapy.
Re-resection may be reasonable after an initial excision or at the time of initial surgery if there is microscopic residual disease or the histopathology was unexpected. In select cases, after chemotherapy, a second look surgery may be helpful.
Lymph node sampling recommendations vary by site. GU and extremety RMS tend to metastasize to nodes more frequently and have a high incidence of node involvement. The high frequency of nodal spread in the limbs, has both prognostic and therapeutic implications. These nodes often do not enhance on imaging. COG currently recommends surgical staging of nodes in extremety RMS disease and for males > 10 with para-testicular disease.
Chemotherapy
Chemotherapy was originally used (prior to 1960s) for mets. Vincristine, actinomycin-D and cyclophophamide were shown to have activity either with vincristine and actinomycin, or the 3 drug combination. Addition of adjuvant VA/VAC chemotherapy to total or subtotal resection disease increased survival from 10-40% to 60-80%. The IRS studies demonstrated that chemotherapy is effective and that a 4 agent regimen may be more effective than 3 agents. Presently studies are looking at VAC/VI (vincristine, actinomycin-D, cyclophosphamide/vincristine/irinotecan) ror intermediate risk patients, and VAC/VA for low risk patients. The goal of this trial is to start radiation sooner than earlier trials (week 4) and to determine if irinotecan can be used as a radiosensitizing agent.
For the moment, VAC is the present standard for RMS patients, with VA only for lowest risk patients.
Radiation Therapy
Radiation goals, like surgery is to provide local and regional control with or without surgery, in combination with chemotherapy.The optimal multimodal strategy is to coordinate RT with surgery and cheomotherapy so as not to impair would healing or drug administration. Major considerations include primary site, extent of surgery, RT-chemotherapy interactions.
Present efforts have looked at reducing dose and volume from original doses and volumes of the 1940s-1950s. 50-65Gy was originally used to very wide margins due to the infiltrating nature of RMS. These techniques achieved 90% local control, but at a cost of significant morbidity. With the addition of chemotherapy, the studies focused on the possibility of reduced-dose, reduced volume feasibility. These studies are summarized below:
| Clinical Group | Chemotherapy Regimen | Conventional Radiation Therapy | 5-Year Survival (%) | Entire Group Survival (%) |
|---|---|---|---|---|
| I | VAC 2 years VAC × 2 years |
NO Yes |
93 81 |
83 |
| II | Cyclic sequential VA × 1 year VAC × 2 years |
Yes Yes |
73 70 |
71 |
| III | Pulse VAC × 2 years Pulse VAC + Adr × 2 years |
Yes Yes |
53 51 |
52 |
| IV | Pulse VAC × 2 years Pulse VAC + Adr × 2 years |
Yes Yes |
14 26 |
21 |
| Overall | 55 |
The results of the IRS-I trial which omitted radiation in completely resected, favorable histology disease, did not demonstrate a survival advantage to adding radiation therapy. The subsequent trials examined doses and volumes of disease. For low volume disease, doses were reduced to 40 Gy in younger children older children received 45 Gy, while those with disease > 5 cm continued to receive 50 Gy - 55 Gy. Unfavorable histology was found to have a higher percentage of failures in group I disease, despite complete resection. In particular alveolar histology was unfavorable to the omission of radiation. Radiation doses < 40 Gy were also noted to be associated with increased rates of local-regional relapse.
The IRS III then segregated by histology as well as otßher factors, distinguishing between unfavorable (alveolar, anaplastic, monomorphous cells) and favorable histologies and the primary site. Postoperative radiation was delivered to all but favorable histology and complete resection (Group I) and Group III with "special pelvic sites" and complete pathlogic response to chemotherapy. Radiation doses were 41.4 Gy for Group I and II patients. For patients with residual disease, a variety of doses were given:
- Younger than 6 and tumors < 5 cm: DOSE= 41.4 Gy
- Older than 6 OR tumor ≥ 5 cm: DOSE=45 Gy
- Older than 6 AND tumor ≥ 5 cm: DOSE=50.4 Gy
| Risk Subgroup | Treatment | 5-Year Progression-Free Survival (%) | 5-Year Survival (%) | Progress (IRS-II vs. IRS-III) |
|---|---|---|---|---|
| Group I (favorable histology) | VA × 1 year | 83 | 93 | C not necessary |
| Group II (favorable histology) | VA × 1 year + RT VAdrA × 1 year + RT |
56 77 |
54 89 |
Need for Adr not proven because of small patient numbers and different histologies compared with IRS-I |
| Groups I and II (unfavorable histology) | VAdrC−VAC + CDDP × 1 year + RT | 71 | 80 | Better than IRS-II because of intense chemotherapy |
| Group II (paratesticular) | VA × 1 year + RT VA × 1 year + RT | 81 | 81 | C not necessary |
| Groups II and III (orbit and head) | VA × 1 year + RT | 78 | 91 | C not necessary |
| Group III (except special pelvic, orbit, and head sites) | VAC × 2 years + RT VAdrC–VAC + CDDP × 2 yeara+ RT VAdrC−VAC + CDDP + VP-16 × 2 yeara+ RT |
70 62 56 |
70 63 64 |
Differences not statistically significant but better than IRS-II because of intense induction chemotherapy, second-look surgery |
| Group III (special pelvic sites) | VAdrAC−VAC + CDDP + VP-16 × 2 years + RT + surgery | 74 | 83 | Better than IRS-II because of intense chemotherapy, early RT, second-look surgery; the bladder salvage rate more than doubled (60% vs. 25%) |
| Group IV | VAC × 2 years + RT VAdrAC-VAC + CDDP × 2 years + RT VAdrAC−VAC + CDDP + VP-16 × 2 years + RT |
27 27 30 |
27 31 29 |
No significant differences and no better than IRS-II |
A, actinomycin D; Adr, doxorubicin; C, cyclophosphamide; CDDP, cisplatin; DTIC, imidazole carboxamide; RT, radiation therapy; V, vincristine; VP-16, etoposide.
Radiation Therapy Treatment Planning And Techniques
Radiation therapy is indicated in all but the most favorable situations:
- Clinical Group I: fully resected disease confined to organ of origin
- Stage I: favorable sites
- Chemotherapy used
- Favorable histology
In this setting, with all of the above favorable prognostic features, the OS5 was 93% and radiation did not appear to offer a survival advantage over surgery and VAC alone. All others are offered radiation. This includes children as young as 24 months, as SIOP has demonstrated a substantial benefit to radiation.
Volume and Technique
There is debate on volume. The COG/SIOP recommends defining the GTV as the pre-chemotherapy. The CTV=GTV+1 cm. Some centers (UPenn) advocate two GTVs: GTV1 = pre-chemotherapy volume and CTV1=GTV1 + 1 cm → GTV2=post-chemotherapy volume, CTV2=GTV2+1 cm. The first (larger) volume is taken to 36 Gy with a boost to 50.4 Gy to the second volume (14.4 Gy boost). 3D conformal is preferred if organ sparing can be accomplished, to avoid spraying low dose radiation to uninvolved areas, as is common with IMRT. Late effects of radiation include second malignancies and these patients have a long life expectancy.
Radiation recommended volumes (mostly IRS-V/VI Trial)
- GTV = presurgical, pre-chemotherapy plus mets at diagnosis
- CTV = GTV + 1 cm
- If planned dose is 50.4 Gy, then reduce CTV to GTV + 0.5 cm after 36 - 41 Gy
- If LN+ then include the entire chain
- If Orbit then do not extend CTV beyond the orbit
- If mass effect the displaced tissue volume should not be covered.
- PTV = CTV + 0.5 cm
Dose: Low risk/Intermediate Risk
Dose is dependent on histology, extent of residual disease and Group.
- Stage 1-3 Group I (completely resected)
- non-alveolar histology: NO radiation
- alveolar histology: 36 Gy
- Stage 1-3 Group II (microscopic residual)
- N0: 36 Gy
- N+: 41.4 Gy
- Stage 1 Group III (biopsy only or subtotal resection)
- Orbit only: 45 Gy
- All others: 50.4 Gy
- Stage 4/Group IV
- 50.4 Gy unless resection meets above Group criteria
- If second look surgery = neg. margin, then reduce dose to 36 Gy
High Risk Disease (metastatic, parameningeal, paraspinal, intracranial extension)
- All patients get 50.4 Gy to primary and metastatic sites EXCEPT orbit which gets 45 Gy
- If > 1 lung met: Whole lung gets 1.5 Gy → 15 Gy, boost residual to 50.4 Gy
- If initial surgery resection margins are negative:
- embyronal histology: NO RT
- alveolar: 36 Gy
- If surgical margins are microscopically positive:
- N0: 36 Gy
- N+, or micrscopic residual: 41.4 Gy
Dose LIMITS:
- Optic Nerve/Chiasm 46.8 Gy
- Lacrimal Gland 41.4 Gy
- Small Bowel and cord: 45 Gy
- Lung V18 < 50%
- Kidney < 14.4 Gy
- Liver whole liver <: 23.4 Gy
- Heart < 36 Gy
Timing
If imminent harm: Day 0 is ok. Otherwise, for VI/VDC/IE/VAC start at week 20 for high risk disease, Intermediat risk disease, Start at week 4 (for time to get a good plan), for low risk start at week 13 (VA or VAC chemotherapy)
Site Specific Recommendations
Orbit
The orbit is a favorable site. It is usually recognized promptly, there are few lymphatic pathways, and most tumors in this site have favorable embryonal histology. Hematogenous spread is infrequent. In the 10% who have alveolar histology, the prognosis is worse.
Surgical management of orbits is no longer commonly done other than to obtain a biopsy specimen to obtain the diagnosis. Generally, any surgical procedure that will compromise vision should be avoided. Orbital exenteration was commonly used but rarely acheived local disease control and the surgery was abandoned in the 1960s. Radiation was noted to achieve significantly better local control after biopsy only. Treatment now consists of biopsy (Group III), chemotherapy and radiation therapy.
Chemotherapy
SIOP attempted to omit radiation therapy, but this study clearly showed worse outcomes with a significant compromise in local control, but no difference in overall survival. Radiaton to the orbit does cause late toxicities. Radiation dose is presently recommended at 45 Gy with chemotherapy intensification for alveolar disease. Treatment is with VAC (vincristine, actinomycin-D, cyclophosphamide) or VA (vincristine, actinomycin-D only) with radiation therapy commencing between weeks 3 and 12. Usually VA is used for embryonal variants and VAC is used for alveolar variants.
Radiation Therapy Treatment Planning And Techniques
Because of the severe late side effects of radiation, 3D-CRT should always be used. 45 Gy should be delivered to the GTV while protecting the organs at risk: lens, lacrimal gland, cornea, retina, optic nerve, orbital bones and brain. Treating with the eye open to avoid bolus effect of the eyelid on the lens may be preferred. Protons may permit greater sparing of normal structures.
Head and Neck: Parameningeal Sites (unfavorable site)
Non-orbtital RMS of the head and neck is grouped into paramenengeal sites and non-parameningeial sites, largely historically due to the difficulty of surgical resection of parameningeal sites. The parameningeal sites are
- the nasopharynx
- nasal cavity
- paranasal sinuses
- middle ear
- infratemporal fossa
- pterygopalatine fossa
Parameningeal RMS represents the majority of non-orbital head and neck RMS. They arise adjacent to the meninges and have the ability to extend along the meninges intracranially and from there, throughout the CSF. Since parameningeal tumors can spread into and penetrate the meninges, CSF should be obtained and analyzed for parameningeal sites. When the initial site is unclear and there is a possibility that there is parameningeal involvement, then This should be considered as though it was parameningeal and CSF should be obtained.
Surgery
Parameningeal sites can rarely be resected without significant morbidity and poor cosmesis. Surgery is generally limited to obtaining a biopsy specimen. Neck dissection is morbid, and the vast majority who have attempted resection end up with Group III disease. If there are suspicious nodes, it is reasonable to obtain them for biopsy.
Chemotherapy
Prior to chemotherapy, the outlook for parameningeal sites was dismal. Survival was 20% with surgery and radiation. With chemotherapy and radiation, the timing and volume of radiation is critical. Radiation volumes have been modified based on information from the IRS studies.
Evolution of Radiation Fields
In IRS-I the initial fatality rate was 90% with radiation delivered to tumor GTV + 2 cm margin at week 6. Because of the poor outcome, this was modified to WBRT v. CSI, with CSI chosen due to the high rate of leptomeningeal involvement within 6 months of treatment (35%). Timing was moved up to week 0 of treatment and spinal RT at week 6. Intrathecal CT was also delivered. This did not change the risk for leptomeningeal disease and CSI was dropped in IRS-III.
In IRS-III, if there was no evidence of intracranial extension, CSF cytology was negative and bone erosion and CNS palsies were absent, then the primary lesion was irradiated with a 5 cm margin, including adjacent meninges. Otherwise, patients got intrathecal chemotherapy. If CT/MRI dmeonstrated intracranial disease, not contiguous with the primary, then WBRT was offered if the CSF was negative.
IRS IV reduced the radiation margin to 2 cm and only those with diffuse intracranial meningeal extension or multiple intraparenchymal lesions were treated with WBRT. Radiation was now given at the beginning of the course of treatment. Radiation was omitted for completely resected, favorable histology disease and intrathecal chemotherapy was abandoned altogether, along with CSI. Surgical extent was limited by cosmesis and function. IRS V continued this tradition.
Radiation Therapy Treatment Planning And Techniques
The radiation therapy treatment volume defining the GTV is the pre-chemotherapy volume. ARST-0531 defines the CTV = GTV + 1 cm and continues to define the GTV as the pre-chemotherapy volume. The study does allow a field size reduction after 36 Gy to the post-chemotherapy volume. All radiation starts at week 4 to allow treatment planning time. CT based conformal planning is required. Radiation is necessary even for very young children, despite the reluctance (per SIOP, which showed a substantial benefit to radiation). Radiation should not be omitted, even in children as young as 24 months old. Advanced techniques such as proton beam and IMRT can provide improved sparing of normal tissues.
Dose
Currently SIOP and COG dictate no dose reduction and recommend 50.4 Gy for both alveolar and embryonal disease. Chemotherapeutic dose intensification is recommended for alveolar disease. Some centers treat the pre-chemotherapy volume (GTV+1 cm) to 36 Gy followed by a field reduction to the post-chemotherapy volume (GTV2+1 cm). This is presently not endorsed.
Head and Neck Non-Parameningeal Sites (favorable)
Non-parameningeal sites are generally favorable, with embryonal histology predominating. Cheek and scalp lesions have a higher frequency of alveolar histology, but superficial lesions may be resectable with wide local excision. Often the best cosmetic outcome is a combination of surgery and radiation therapy. For deeper tumors involving the oral cavity, buccal mucosa, larynx, parapharyngeal or parotid regions, radiation is generally necessary. As of IRS-V these tumors are classified as Stage I (favorable site) or Stage II (unfavorable site, small disease) and may receive less intense chemotherapy (VA).
Bladder and Prostate (Unfavorable)
Natural History and Epidemiology
Pelvic tumors are divided into anatomic subgroups because natural history of each site, treatment and prognosis are different. Some children present with locally advanced disease of uncertain primary site. These large tumors are associated with an unfavorable prognosis.
Bladder and prostate primary tumors account for about half of all pelvic RMS. 75% present before age 5, and more than 90% are embryonal, and 1/3 of those are botryoid type. Because the disease often involve both sites, it is often difficult to determine a bladder versus prostate origin in boys. Patients with disease originating in the prostate have an inferior survival.
Historically survival with anterior pelvic exenteration, combined with chemotherapy and radiation therapy for microscopic (Group II) or gross residual (Group III) disease has been associated with survival rates of 70%. With bladder preservation, a focus has been on reducing the amount of surgery required to preserve bladder function. In early studies this approach failed and resulted in a reduction in survival to 55%.
Subsequent studies (IRS-III) intensified therapy with planned radiation at 6 weeks after start of CDDP/Adriamycin chemotherapy. OS5 was 82% with 64% preserving a functioning bladder. This study demonstrated the need for early radiation therapy. The IRS-IV study confirmed this with 40% reporting a normally functioning bladder, with maintenance of continence in 69% treated with conservative surgery.
The German Weichteilsarkom Studiengruppe protocol treated with multi-agent chemotherapy followed by response modified radiation and surgery.
- Complete resection: No radiation
- Others: 32 Gy to 44.8 Gy at 1.6 Gy BID depending on IRS group and Chemo response.
5 year Event Free Survival was 70%.
Pathology
About 80% are embryonal, and of those, most are botryoid.
Clinical Workup and Evaluation
RMS in prostate/bladder tends to occur in very young children, most < 5 years old. Urinary abnormalities including dysuria, polyuria, incontinence, and urinary retention are early signs. Regional nodal metastases is not uncommon, with documented ranges of 20-30% to the hypogastric or external iliac nodes. Only 15% have demonstrated distant mets at diagnosis.
Imaging Workup
Ultrasound is the first imaging study, followed by thin slice CT and/or MRI of the abdomen and pelvis. These studies allow greater detail to evaluate the lymphatics and the primary mass. This study is used as a baseline to assess response to subsequent treatment. PET/CT is being increasingly used to evaluate lymphatics and assess for distant metastases. CT of the chest should be performed to evaluate the chest for hematologous spread.
Cystoscopy with Biopsy
Endoscopic biopsy may allow for diagnosis. If adequate tissue cannot be obtained, then an open biopsy will provide adequate tissue. If urinary retention is present, then a ureteral stent can be placed or, alternatively urinary diversion.
General Management and Treatment
High survival rates are evident, but at a significant cost of morbidity and subsequent quality of life. Disease Management has been evolving from pelvic exenteration to less aggressive, organ-preserving therapy while maintaining high survival rates. Multimodality goals are to cure with bladder function preservation.
Surgery
Up front surgery is no longer performed, except for tissue diagnosis. If the bladder cannot be preserved, up front, then surgery is deferred in favor of chemotherapy and radiation therapy that will hopefully allow an organ preserving surgery. IRS-III added routine radiotherapy after chemotherapy week 6 except for those whose tumor could be completely excised with bladder preservation, followed by surgery to remove residual tumor. Bladder preservation was 60% at 4 years and survival was 90% for patients presenting with local only or regional disease.
IRS-IV recommended alkylating agent based chemotherapy and radiation therapy. At 6 years, OS was 82%, FFS was 77% and bladder preservation was 70%
Radiation Therapy Treatment Planning And Techniques
3D conformal treatment planning is essential. CT (for boney anatomy and growth plate identification) fused with MRI (for soft tissue identification) provides the best imaging technique. For patients with large tumors displacing bowel GTV is defined as the preoperative tumor volume excluding the debulked portion while accounting for shifting structures.
Critical structures are:
- bladder
- rectum
- bowel
- pelvic and femoral head growth plates (keep dose < 10-20Gy)
- penile bulb and testes
IMRT may be disadvantagous over 3D crt due to the larger volume of low dose irradiaiton which may increase the risk for second malignancies.
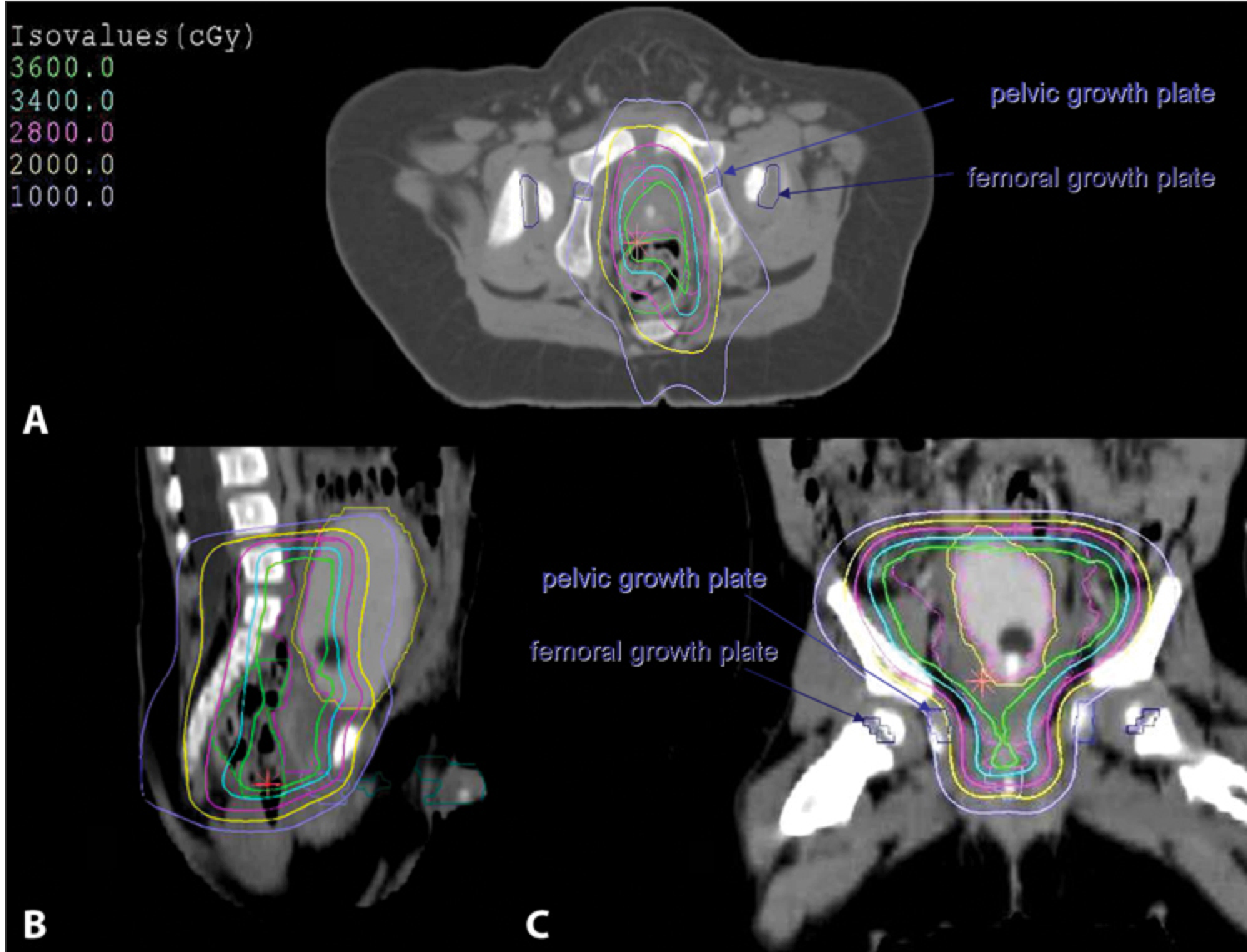
Paratesticular RMS
Epidemiology, Pathology and Natural History
These represent about 7% of all RMS and mau arise anywhere along the spermatic cord. The tumor is usually a painless testicular mass that does not transilluminate. This is usually found early and amenable to complete resection which is associated with 90% cure rates. Histologically, nearly all of these are embryonal.
The recommended surgical treatment is inguinal orchiectomy and if there is no invasion of scrotum, and proximal spermatic cord has a negative margin, then surgical treatment is considered definitive. Surgery is inguinal orchiectomy with high cord ligation.
Lymph nodes are around 14% in children < 10 years old, but are 47% positive in children ≥ 10 years old. EU does not recommend retroperitoneal staging, treating with intensified chemotherapy in high risk patients and salvage radiation. In the IRS high risk subsets, the recommendation of ipsilateral RP nerve sparing node dissection for all children ≥ 10 years old. Alternatively, node enlargement on thin sliced CT imaging is considered evidence of nodal disease.
Nodal disease is a poor prognostic indicator with survival at 69% for N+ compared with 96% in node negative disease. Regional radiation is recommended to the para-aortic and ipsilateral iliac chains if there is evidence of nodal disease. Surgical violation of the scrotum is reason to recommend hemiscrotectomy or (less commonly) radiation after orchioplexy to avoid irradiating the remaining testicle.
The most common indication for post-surgical radiation therapy in paratesticular disease is lymphatic involvement.
Vaginal and Vulva
Epidemiology, Pathology and Natural History
RMS of the vagina and vulva are rare, around 3% of pediatric RMS. The majority of these are vaginal and are botryoid. They occur in very young children, 90% < 5 years with a mean age of 2 years for vaginal primaries. Presenting symptoms include vaginal discharge and bleeding, and a protruding vaginal mass. They commonly arise from the anterior wall, are frequently multi-centric and can invade the vesico-vaginal septum or bladder wall. Node involvement is uncommon.
These tumors are almost exclusively embryonal and are of the botyroid variant. Vulvar RMS is even less frequent than vaginal RMS. It frequently presents as a firm nodule in the vulva. Radiation referral should take place before treatment as these tumors respond rapidly to chemotherapy and pre-treatment physical findings are important to treatment planning.
Chemotherapy, despite the rapid initial responses is inadequate alone. There are high rates of local recurrence if surgery or radiation is not added to the treatment. If surgery is used, it is most commonly used for biopsy and tissue diagnosis, unless surgery can be achieved to negative margins without cosmetic and functional morbidity. Current COG guidelines recommend radiation therapy in all patients with post-surgical microscopic or macroscopic tumor.
Survival is excellent at 90% for infants and adolescents.
Extremety (unfavorable)
Epidemiology, Natural History and Pathology
Extremety RMS comprises about 20% of pediatric RMS cases. Tumors in this location continue to have a poor prognosis compared to other sites. Treatment intensification has not changed this. OS3 has improved from 47% in IRS-I to over 70% in IRS-III and IV. However, relapses requiring salvage remain high. FFS3 in IRS-IV was only 55%.
These tumors are frequently alevolar or undifferentiated subtypes, they are large and deeply invasive. They are associated with a high probability of lymphatic and hematogenous mets. Complete (Group I) surgical resection is difficult to achieve, and frequently requires extensive dissection. There is a high risk of residual disease (Group II/III). Surgery also has high functional and cosmetic morbidity.
General Management and Treatment
Chemotherapy and multi-agent chemotherapy provide excellent local control, which permits the avoidance of extensive surgical procedures in favor of limb salvage treatment. There is no evidence that amputation is more often curative than wide local excision. Amputation could be considered if limited excision and high dose irradiation would produce unacceptable functional outcomes. Intensive physical therapy should accompany radiation therapy, before, during and after treatment to minimize the functional impact. Amputation is indicated in patients with distal alveolar extremety lesions where gross reoval is otherwise impossible and in patients with massive recurrence despite earlier conservative therapy. The preferred surgical management is wide local excision with negative margins.
Lymphatic involvement was investigated in IRS IV with surgical staging. 50% were found to have lymphatic involvement. Of those that had clinically negative nodes, 17% had pathologically microscopic lymph node involvement. PET/CT has been explored to evalute the utility in staging RMS of the extremety, which is a topic of current COG protocols. Additionally Halperin suggests that consideration of exploring the role of sentinel node biopsy is reasonable in future studies. Current COG studies require surgical node sampling for staging.
Chemotherapy is always given as a component of extremety RMS.
Radiation therapy's role is dependant on Group Staging and is omitted only for completely resected, favorable histology tumors with negative nodes. (Note: this is the IRS I/III, favorable/favorable recommendation).
Radiation Therapy Treatment Planning And Techniques
Volumes
RT is only omitted for fully resected, favorable histology disease. All others get treated to the pre-treatment GTV with a 1.5 cm margin per current COG protocols. A half cm margin on the CTV for PTV construction is used. A strip of skin should be spared to prevent severe lymphedema. 3D conformal techniques using opposed fields generally result in optimal dose distribtution. Joint sparing and epiphyseal plate sparing should be considered where possible.
Dose
Group I alveolar disease should get 36 Gy (per IRS-V), residual disease gets 41.4 Gy (microscopic and surgical N+), large disease and residual disease gets 50.4 Gy.
Outcomes, Patterns of Failure, Prognostic Indicators
Side Effects and Complications of Treatment
Outcomes, Patterns of Failure, Prognostic Indicators
There are four histologic types of RMS with prognostic indications: embryonal, alveolar, pleomorphic and mixed. The International classification system divides RMS into favorable and unfavorable types, with central pathologic review for COG study patients.
| Frequency (%) | Actuarial 5-Year Survival (%) | |
|---|---|---|
| Superior prognosis | ||
| Botryoid rhabdomyosarcoma | 6 | 95 |
| Spindle cell rhabdomyosarcoma | 3 | 88 |
Intermediate prognosis |
||
| Embryonal rhabdomyosarcoma | 79 | 66 |
Poor prognosis |
||
| Alveolar rhabdomyosarcoma | 32 | 54 |
| Undifferentiated sarcoma | 1 | 40 |
| Other | 9 |