Embryonal Tumors
Medulloblastoma
Embryonal tumors are the second most common pediatric tumors. (Low grade gliomas are the first and ependymomas are the third). These tumors include:
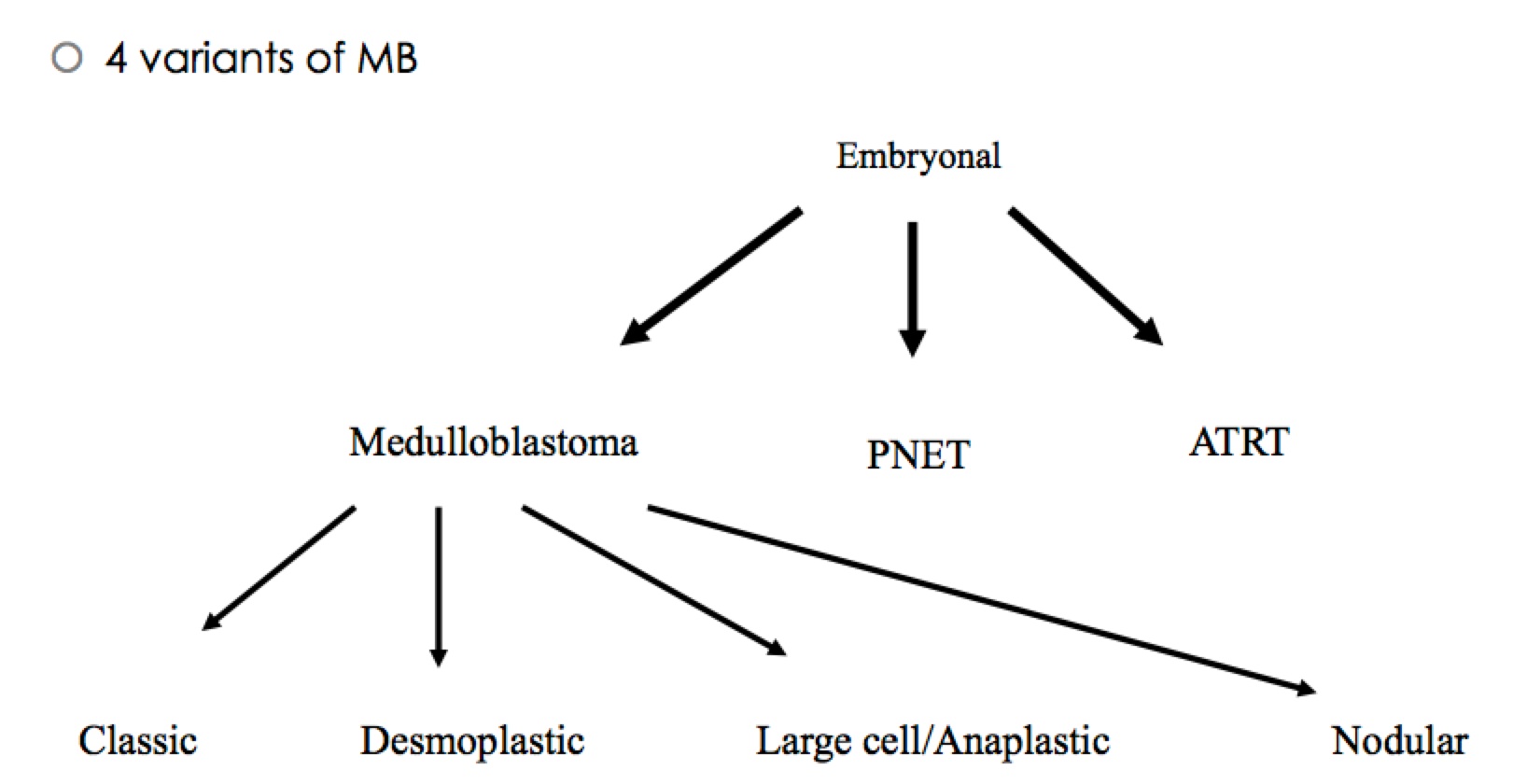
- Medulloblastomas
- desmoplastic or nodular medulloblastoma
- medulloblastoma with extensive nodularity
- anaplastic/large cell medulloblastoma
- Supratentorial PNET (sPNET)
- CNS neuroblastoma
- medulloepithelioma
- ependymoblastoma
- Atypical teratoid/rhabdoid tumor
In short, a bunch of small little round blue cell tumors.
Pathology
Simply stated: 4 variants arising from the same family as PNET adn ATRT from embryonal origins
Epidemiology and Natural History
Medulloblastomas account for 15% - 20% of pediatric tumors with a median age of presentation of 6 years. It is usually a malignant invasive tumor of the cerebellum with predominantly neuronal differentiation and an inherent tendency to metastasize via CSF pathways. Most arise in the vermis and project into the fourth ventricle. Patients typically present with signs of increased ICP. On MR they appear as solid masses with homogeneous enhancement on T1 gad series. Leptomeningeal seeding runs about 30% to 35% and the entire neuro-axis should be imaged and CSF sampled for cytology. Medulloblastomas can spread outside the CNS to nodes and bone.
Risk Groups
Medulloblastomas are subdivided into standard risk and high risk categories.
- Standard Risk: complete resection or residual disease < 1.5 cm2 on 48-72 hour post-op MRI, CSF negative, and non-anaplastic/large cell
- High Risk: anything not standard risk, M1+, > 1.5 cm2 residual, non-cerebellar primaries
Staging: Chang
The key staging component in medulloblastoma is the M stage. T stage is defined, as tumors < 3 cm diam (which corresponds with 1.5 cm2 residual --2 √1.5 cm2=1.2.45 cm ), but M stage determines therapy, for the most part.
| Stage | Description |
|---|---|
| M0 | No evidence of gross subarachnoid or hematogenous metastases |
| M1 | Microscopic tumor cells in CSF |
| M2 | gross nodullar inctracranial seeding beyond primary site in crebellar/cerebral subarachnoid space or in third or lateral ventricle |
| M3 | Gross nodular seeding in spinal subarachnoid space (drop mets) |
| M4 | extra CNS metastases |
Clinical Workup and Evaluation
Clinical workup consists of an history and physical exam for increased ICP signs and symptoms as well as ataxia, morning nausea and somnolence. MRI imaging is essential and given the high propensity for spread (30-35%) along the CNS axis, should cover the brain and spine. Once MRI has demonstrated no increased intracranial pressure, an LP for CSF analysis (cytology, glucose) should be performed to see if there is metastatic disease. If it is unsafe to proceed pre-operatively to LP, then the LP should wait a minimum of 2 weeks to insure there are no false positive. A post-op MRI should be obtained at 48-72 hours post-op to determine the volume of residual disease. Residual disease > 1.5 cm2 moves patients from standard risk to high risk.
- History and Physical with consideration for increased ICP due to 4th ventricle blockage (headache, nausea, confusion, diminished pupillary response).
- MRI brain followed by MRI of the CNS axis.
- If MRI demonstrates increased ICP, delay LP until after surgery.
- Surgery for therapeutic debulking and tissue.
- Goal of surgery is maximum safe resection
- Goal of surgery is to achieve < 1.5 cm2 of residual disease.
- Post-op MRI brain within 48 hours of surgery
- MRI of the spine at POD 10-14
- CSF (LP) at POD 10-14
General Management and Treatment
Standard Risk Disease (Complete resection < 1.5 cm2 residual, CSF and spine imaging negative, non-anaplastic/large cell)
Best possible surgical resection should be performed. Staging is done both pre-operatively and post-operatively. Pre-operative imaging of the Craniospinal axis followed by LP (if safe) to determine the extent of metastatic disease, if any. If an LP cannot be done pre-operatively then it should be delayed until 3 weeks post-op to avoid false positives. Post-operative MRI should be performed at 48-72 hours to determine residual disease.
ALL patients with medulloblastoma get CSI! Most get chemotherapy too. If chemotherapy is used, then the CSI dose is reduced, but the GTV dose remains the same. Generally vincristine is used concurrently with radiation and is followed by vincristine, cyclophosphamide and cisplatin (PCV).
Infants
Medulloblastoma accounts for 20% - 40% of all CNS tumors in infants. Half have favorable histologies, but the overall prognosis is worse than in older children. The rates of complete resection is lower, the frequency of leptomeningeal disease is higher, and treatments may be sub-optimal. There are significant risks to neurocognitive development with the delivery of radiation to babies and very young children. Chemotherapy has been used in an attempt to delay the need for radiation or avoid it altogether. Infants who have a complete resection followed by chemotherapy and M0 disease have been reported by POG to have an OS5 = 69% and by Germany to have 93%. The German study added intrathecal methotrexate, which can also contribute to neurocognitive sequelae.
To date, most studies with the possible exception of very young children with very favorable histologies and resection (desmplastic/nodular meddulloblastoma without residual disease) demonstrate the need for radiation therapy as an important component of treatment. Recurrences are mostly early (6 - 12 months) and local. A North American study used early radiotherapy to a limited treatment volume (CTV= GTV[tumor bed]+1 cm) in patients without leptomeningeal spread. The UK/SIOP studies demonstrated an OS5 of 52.9% with favorable histologies (desmoplastic/nodular), but the other histologies do not do nearly as well. At present there is strong impetus to avoid radiation and especially CSI in very young children for as long as possible.
Resection Definitions
The following resection definitions have been used:
| Description | Characteristics | |
|---|---|---|
| GTR | Gross tumor resection | No evidence of residual disease, negative post-op imaging |
| STR | Subtotal resection | 51% - 90% resection|
| NTR | Near total resection | > 90% resection estimated by surgeon, < 1.5 cm2 residual on post-op MRI (POD 2) |
| PR | Partial resection | 11-50% removed. |
| Biopsy | Biopsy | < 10% removed |
Radiation Therapy Treatment Planning And Techniques
Standard Risk Medulloblastoma
Radiation therapy is always recommended to the craniospinal axis. The dose is dependent on chemotherapy. The present recommended dose is 23.4 Gy to the CSI with concurrent vincristine. Adjuvant chemotherapy follows with cisplatin 75 mg/m2, cyclophosphamide and vincristine 1.5 mg/m2 (PCV). This is followed by a boost to the posterior fossa to 55.8 Gy. Survival rates at 4 years now approach 85%. Ongoing trials are testing a lower dose of 18 Gy to the CSI with a reduced volume to the posterior fossa, but this is not recommended off trial.
Standard Risk
Standard risk is residual < 1.5 cm2 on 48 hours post-op MRI, negative CSF at 10 days post-op, non-anaplastic disease.
The addition of CDDP chemotherapy is assocated with improved EFS5yr to about 80%. If CDDP is added to chemotherapy, the CSI dose can be reduced from 36 Gy to 23.4 Gy. The chemotherapy regimen is weekly vincristine on radiation followed by PCV chemotherapy (vincristine, cisplatin, and cyclophosphamide).
- If CDDP is used, then CSI RT to 23.4 Gy (otherwise use 36 Gy)
- Follow with a boost to the posterior fossa to 36 Gy and
- boost to the primary site to 55.8 Gy (PTV=CTV (post-op cavity) + 2.5 cm margin)
Surgery → Radiation with concurrent vincristine (weekly) → vincristine, cisplatin and CCNU.
Event Free Survival at 5 years was 83%.
CSI technique
Craniospinal irradiation technique is a technically complex technique which requires matching of divergent beam angles to insure proper irradiation without overdose/underdose to key regions of interest. There are three potential beam divergences that must be accounted for and corrected. These include a spine superior and inferior field, generally delivered using a PA field. Next the cranial portion of the field is delivered with opposed laterals which must be adjusted to accommodate beam divergence from the collimator in the lateral plan and in the PA plan. Thus there must be both a colimator rotation and a table rotation to provide match lines in these two planes. In additin there must be a skin gap light field separation calculation to insure there is no overlap/underlap on the cord area from the two abutting fields. Here are the setup instructions:
- Setup patient face down on the prone pillow with an aquaplast mask to immobilize the head. Anesthesia/sedation may be necessary.
- CT imaging at 2.5 mm cuts from well below the bottom of the thecal sac through the top of the cranium for treatment planning purposes.
- Define the Spinal Field first:
- The inferior border is the bottom of the thecal sac as seen on fused MRI around S2
- The superior border is defined as low as possible while clearing shoulders to avoid exit dose through the mouth and brain. Usually this is C5/C6 region.
- Plan on feathering the edges once a week (or about every 9 Gy) by about a cm.
- Define the lateral borders at 1 cm lateral to the vertbral body with possible wider coverage of the sacral nerve roots (spade)
- Define the Whole Brain fields:
- Rotate Collimator to match the divergence of the spine field (tan-1((L/2)/SSD))
- Rotate the couch toward the gantry to avoid divergence into the spinal field (tan-1(L/2/SAD))
- Adjust the gantry to avoid divergence into the contralateral lenses and retinas.
- If the spine field is too long for the accelerator, consider using an extended SSD. If this doesn't work, then two fields setup so that the match point is deep (anterior) to the cord, calculate a gap setup and feather the beam weekly or about every 9 Gy.
Volumes
- CTV - Post. fossa
- Inferior margin is set to C1, the superior margin is set to the tentorium, the lateral margin is the bones of the occiput and temporal bones.
- PTV
- PTV expansion on the CTV should be 3-5 mm margin and should extend to cover the posterior clinoid and C2.
High Risk Medulloblastoma
High risk medulloblastoma is disease with any of the following: post-operative residual disease > 1.5 cm2 on 48-72 hour post-op MRI, positive CSF either pre-operatively or at 3 weeks post-operatively, leptomeningeal spread by MRI, or anaplastic/large cell disease.
Much of the present research has focused on chemotherapy which has resulted in significant improvements in DFS. Risk factor definitions have changed over time, as have the therapies, making it more difficult to do head-to-head retrospective studies and to compare present management studies. High risk disease is a heterogeneous group, more so than the present Standard Risk group. Retrospective re-assignment of patients from one group to the other based on current assignment protocols has led to improvements in both group's statistics.
Coverage of the entire meninges is necessary, including the areas of the nerve roots and the cribriform plate. SIOP demonstrated 50% of relapses are associated with target definition failures. Relapse rates were 17% in patients who had one are of inadequate coverage, 28% with two sites and 67% with three or more sites of inadequate coverage. CSI is indicated in all (except possibly babies with favorable histologies) medulloblastomas. CSI is followed by a boost to the posterior fossa bringing the total dose to 54-55.8 Gy. Using conformal 3D treatment techniques, dose should be reduced to the organs at risk, in particular, the inner ear and cochlea. This is important in patients who will also be receiving concurrent chemotherapy.
Optimal volumes are still debated, but presently, the posterior fossa is the GTV. The CTV is a 1.5 cm expansion corrected for anatomical barriers is reasonable.
High Risk medulloblastoma is treated similarly to standard risk medulloblastoma except the doses are different.
- CSI dose: 36 Gy
- Posterior Fossa Boost: 54 Gy
- Spinal nodules are boosted to 45 Gy (be sure to watch cord doses)
- Supratentorial PNET adn pineoblastomas are treated as high risk medulloblastomas.
Treatment scheme is based on ACNS0332. For ages 3-21 years, Concurrent VCR with RT or Concurrent VCR+carboplatin with RT followed by CT ± isoretinoin.
Baby POG
Due to the severe neurologic sequalae of radiation to the CSI in babies aged < 3 years, patients are treated with chemotherapy alone until the reach age 3. Then radiation is used. The outcomes are PFS of 40% at 2 years, which compares favorably to surgery + RT. With a GTR, there is a 90% 1 year PFS, which much more normal cognitive development if RT can be delayed as long as possible until after the babies are at least 3 years old.
Delayed radiation therapy is associated with poorer outcomes, but this must be balanced against the substantial morbidity of radiation in infants. CSI should start 28-30 days post-op, in a timely fashion without undue treatment gaps, including holiday breaks. SIOP demonstrated significantly worse outcomes when elapsed treatment time extended beyond 50 days when compared with treatment times over 45-47 days. If CSI must be interrupted, then treatment to the posterior fossa should continue.
Outcomes, Patterns of Failure, Prognostic Indicators
Side Effects and Complications of Treatment
Risks of Surgery
Typical Neurosurgical risks and Posterior Fossa Mutism Syndrome in 15-25%
- meningitis
- CSF leak
- pseudomeningocele
- Mutism
- Dysphagia
- truncal ataxia
- Hypotonia
- increased mood lability
- (infrequently) respiratory failure
PF mutism disrupts the red-nuclei tracts to the supplementary motor cortex. It happens about POD 1-2 and may take months to improve.