Pediatric Low Grade Gliomas
Epidemiology
Low grade astrocytomas are a heterogeneous group of tumors which tend to follow an indolent course in children with OS10 and OS15 of 80% and 90%. They are grouped by anatomic location:
- Cerebellar astrocytomas (25% - 20%)
- Hemispheric astrocytomas (10% - 15%)
- Midline supratentorial tumors including the corpus callosum, lateral and 3rd ventricles, hypothalamus and thalamus
- Optic Pathway tumors
- Brainstem Low Grade Astrocytomas
- Low Grade Astrocytomas of the spinal cord
Pathology
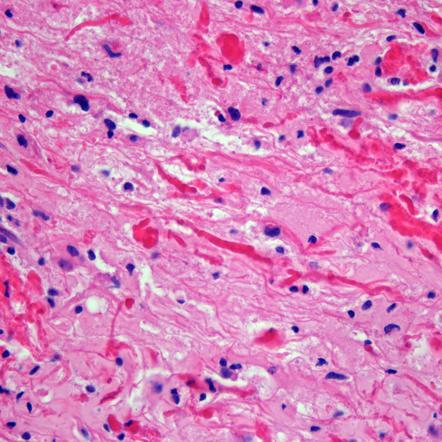
Pilocytic astrocytomas are the most common pediatric type, accounting for almost all lesions of the cerebellum and anterior optic pathway. They accoutn for a smaller fraction arising in the deep midline structures and in the cerebral hemespheres. They are well circumscribed tumors and frequently have a cystic component to them. Histologically they are characterized by biphasic patterns with compacted bipolar cells, with Rosenthal fibers, microcysts and granular bodies. Mitoses are rare. A variant pilomyxoid astrocytoma appears to be more aggressive than a pilocytic astrocytoma.
Diffuse astrocytomas account for about 10% - 15% of all LGAs. They account for a higher percentage in infants and adolescents. Most intrinsic pontine tumors and larger portion arising in the cerebral hemispheres are diffuse astrocytomas. Diffuse astrocytomas grow by infiltration rather than by mass effect and destruction of neighboring tissues. These tumors are usually not well circumscribed. They are composed of fibrillary or pemistocytic neoplastic astrocytes. Cellularity is increased, and there is nuclear atypia. Mitotic activity, necrosis and microvascular proliferation are absent. The growth fraction is usually low (Ki-67 labeling). Diffuse astrocytomas may undergo malignant transformation.
Natural History
Patients with LG astrocytomas present with a long history of non-specific and non-localizing symptoms. Signs and symptoms of increased intracranial pressure may be seen in mid-line and cerebellar tumors. These patients may present with neck stiffness, torticolis, as a manifestation of increased ICP causing tonsillar herniation, altitudinal diplopia or spinal accessory nerve irritation. Seizures are common (75%) in patients with hemisphere lesions. Other signs include:
- focal motor deficit with hemispheric tumors
- visual field disturbances with tumors compressing the optic pathways
- neuroendocrine deficits with hypothalamic tumors
- diencephalic syndrome (emaciation with loss of subcutaneous fat, alert appearance, increased vigor and euphoria, pallor without anemia, nystagmoid eye movements) in young children with chiasmatic/hypothalamic tumors.
Clinical Workup and Evaluation
History and physical exam and CNS imaging are the primary work ups. In particular ask about NF-1. NF-1 patients are predisposed to optic pathway tumors and may have astrocytic tumors in other parts of the CNS. Pilocytic astrocytomas have unique imaging with a well circumscribed mass, frequently with a cystic component that may be large relative to the size of the tumor solid component. The solid component enhances brightly on T1/gad sequences and there is little edema or mass effect. Diffuse of fibrillary astrocytomas are not well seen on non-contrast enhanced CT or T1-MRI. The are seen well on T2/FLAIR imaging which best illustrates the extent of disease.
Imaging
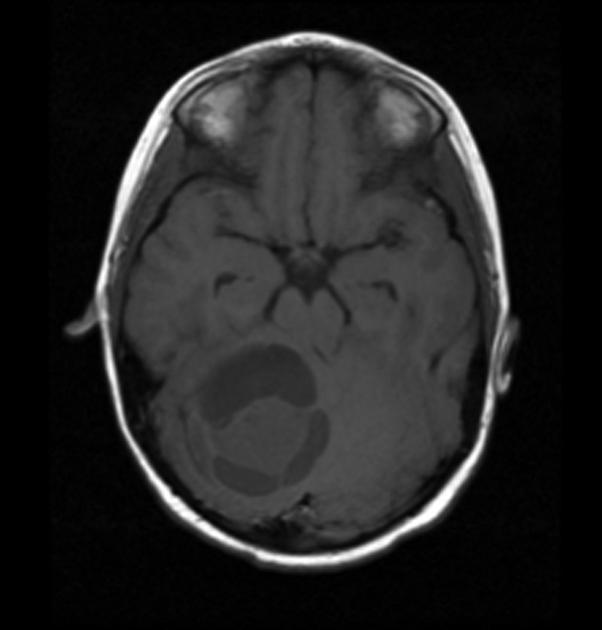
|
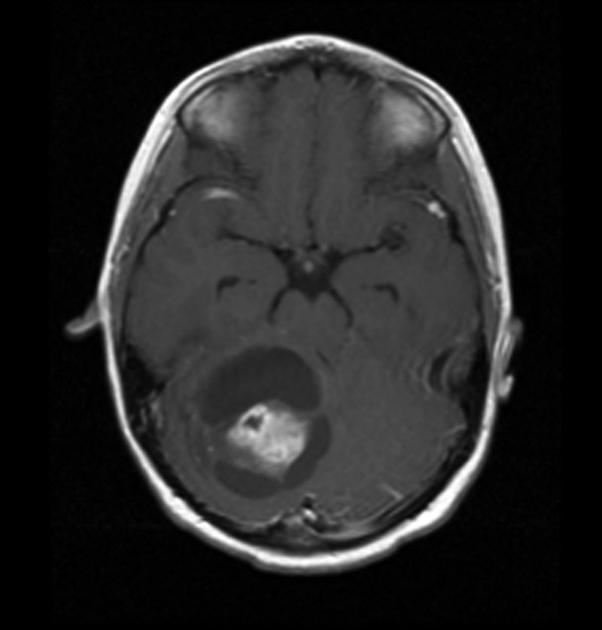
|
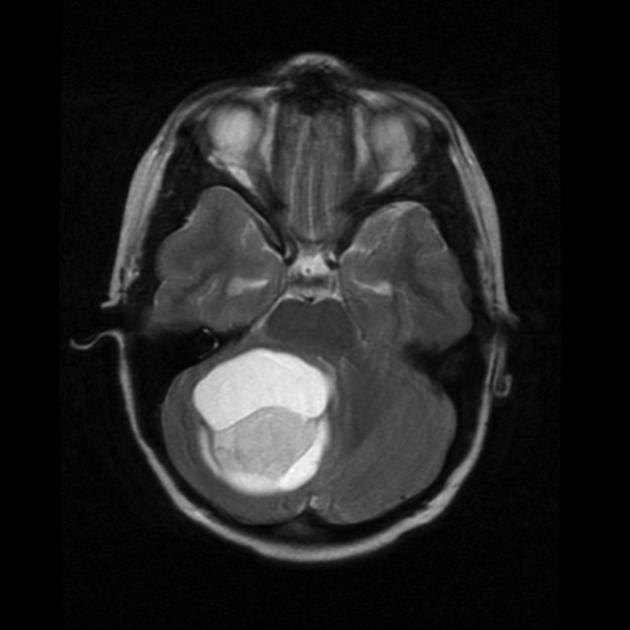
|
General Management and Treatment
Some children with low grade astrocytomas may not require tumor specific treatment. These inlcude patients with NF-1. Tumors may remain stable over long periods of time. These tumors, even if symptomatic may not need immediate treatment other than surveillance. Followup MRI is essential to identify patients with progression manifested by increasing size or gadolinium enhancement. Treatment is usually radiation or surgery at the time of progression and is associated with a high probability of long term control. If there is any doubt about the nature of the lesion, a period of observation is reasonable.
Surgical Managment
Surgical techniques are the mainstay of treatment for LGAs (low grade astrocytomas). Complete resection (R0) is more likely with modern surgical techniques which include computer assisted resections, with intra-operative MRI to permit greater degrees of resection, especially when included with function MRI (fMRI) to help protect eloquent areas. This allows many patients who were formerly considered inoperable to have a sucessful surgery. Complete resection is now achievable in > 80% of cerebral, cerebellar and spinal cord tumors and ~ 50% of tumors of the dienephalon. Children who undergo complete resection do well with long term disease free survival rates of 80% to 100%. Results are better for pilocytic than for fibrillary or diffuse LGAs.
For children who undergo less than complete resection, PFS after surgery alone is less satisfactory. The CCG/POG studied observation in less than complete resection. They found the PFS8 was 56% if the residual disease was < 1.5 cm3 and 45% if the residual disease was > 1.5 cm3. These patients can be salvaged, either with additional surgery or radiation and they have an OS8 of 95% for surgical salvage and 90% for radiation salvage.
The role of radiation following complete resection is reasonable clear: it is not necessary, given the high survival rates and the good success of salvage treatment at recurrence. The role of radiation therapy in incompletely resected disease is less clear. In most studies the use of radiotherapy did show improved DFS, but no survival benefit. Only about half of incompletely resected patients will progress, therefore, at present, the usual recommendation is to defer post-operative radiation in patients who are neurologically stable. These patients should be closely followed with 6 month interval MRIs for the first 3 years, when the risk of progression is the greatest. If there is progression then a second surgery for operable lesions is recommended with radiation reserved for inoperable progressive lesions.
Formerly most, if not all, of those with deep midline or mid-brain tumors, but now, with modern techniques, surgical resection is increasingly possible in these lesions -- up to half of the lesions. The overall strategy should be the same as for other locations, but with the knowledge that the outcomes may not be as satisfactory. mid-line chiasmatic tumors, the outcomes are worse (COG) with PFS8 25% and OS8 of 84%.
Chemotherapy
When adjuvant treatment is indicated, chemotherapy is also an option, which is particularly appropriate for infants and young children and kids of all ages with NF-1. This will permit the delay of radiation treatment to reduce the amount of neurocognitive sequelae. Complete response to chemotherapy is not uncommon, but overall response rates that include stable disease rante from 70% to 100%. the use of chemotherapy has been shown to permit the delay of radiation by between 2 and 4+ years. When any benefit of delay is compromised by further tumor progression, or the need for larger radiotherapy volumes, the benefit from further delay in children age 5 - 8 is uncertain. More recent targeting and delivery advances in radiotherapy has led to increased use in earlier patients, a reassessment that is now ongoing.
Radiotherapy in LGA
Due to improvements in surgery and successful treatment of younger children (< 3 years old) with chemotherapy, radiation has a reduced role in LGA. Only about 10% of children with LGAs now receieve radiation therapy as a component of their treatment.
Indications for radiation:
- Radiotherapy is not indicated after complete resection
- Radiotherapy may be indicated following incomplete resection in situations where tumor progression would compromise neurologic functions (e.g. "threat to vision")
- The clearest indication for radiation in LGA is in patients with progression and/or symptoms in disease that is unresectable.
Radiation Therapy Treatment Planning And Techniques
Volumes
For children who undergo less than complete resection, PFS is less satisfactory. COG (CCG, POG) suggests that the use of radation results in improved DFS but without OS benefit. Only half of the patients will develop disease, the present recommendation is observation using serial 6 month MRI's with second surgical resection as the mechanism of choice. If the recurrence is inoperable, then either radiation or chemotherapy is recommended. For those cases where radiation is recommended, the margins are small, the GTV consisting of imaging visualized tumor on MRI T1/gad series just prior to treatment. The post-operative imaging should be used and should be a smaller volume than the pre-operative imaging. CTV margins are small to none (ie just cover the GTV), unless the disease is an infiltrating diffuse varient. Then the appropriate GTV is any T2 or FLAIR abnormalities, with the CTV expansion = GTV + 1 cm to 1.5 cm.
Dose and Fractionation
EU and North American studies in adults failed to demonstrate advantages to higher doses beyond 50.4 Gy. This has not been confirmed in children, therefore the present practice is reported (2011), 54 Gy - 55 Gy. It has been reported that there are biological differences in pediatric LGA and adult disease. At present the recommendation for a "standard" dose of 50 - 54 Gy depending on the child's age and tumor location with respect to critical normal structures such as the chiasm, and perhaps the hippocampus.
SRS has been used with single fraction doses of 10 Gy - 20 Gy to the LGA. This has not been proven, and may not be appropriate for diffuse infiltrating disease.
Outcomes, Patterns of Failure, Prognostic Indicators
The overall survival for surgically resected tumors is 95% with surgery and 90% with radiation. Most surgical recurrences can be re-resected successfully with radiation reserved fro those increasingly fewer patients that cannot be surgically resected.