Ewings Sarcoma
Anatomy

Epidemiology
Ewings sarcoma is the second most common bone cancer of children in the US. There are about 200 cases per year. (behind osteosarcoma). Boys are more frequently afflicted than girls, and the median age at presentation is 14 with a range of 8-25 years. Asians and Africans are significantly more rare.
Pathology
Ewings sarcoma consists of little round blue cells on H&E stain and PAS positive. The cells are uniformly vimentin positive, indicating neuronal elements. Ewing's sarcoma is associated with t(11;22) and t(21;22) translocations.
Natural History
Ewings sarcoma commonly presents in the lower extremety with femur 15-20%, pelvis 20%, humerus 5%, ribs 9-13%, spine 6-8%. Most present with localized disease but about 20-25% have lung mets, bone mets or bone marrow disease. Nearly all have micrometastases at diagnosis, so ALL get chemotherapy.
Ewings is a family of small round blue cells that stain positively on PAS. They include Ewing's sarcoma (bone = 87%), estra-osseous Ewing's (8%) peripheral PNET (5%), Askin's Tumor (chest wall PNET).
The disease is most commonly found in teens in the metaphyseal and diaphyyeal regions of long bones. It was frequently associated with fever and pain. Ewings is highly radiosensitive.
More than 90% have t(11:22) or t(21:22) transformations. c-myc is frequenly expressed in Ewings, (contrast with neuroblastoma = n-myc.)
Clinical Workup and Evaluation
Ewings commonly presents in teenagers with pain, in 90% of the cases. Local swelling or mass effect related to bone tumor is apparent in the majority of children. A distinct, soft tissue mass can be seen clinically in about 1/3 of the cases. Significant ovement restriction has been seen. Neurologic symptoms occur in 15%.
Workup
Workup consists of biopsy, cytogenetics, CT and MR imaging, including chest CT. Bone scan, ±PET scan, bone marrow biopsy, LDH and routine chemistries. Disease can spread to the lung, therefore imaging of the chest is required.
- CT and MRI of primary disease
- CT of the chest
- Bone Scan
- Bone Marrow Biopsy
- Labs (ESR and LDH)
- Tissue biopsy
- PET recommended, but not required.
Plain film x-rays show a moth eaten lesion in the diaphysis. Lytic lesions are more common than blastic lesions and "onion skinning" may be present for sub-periosteal lesions.
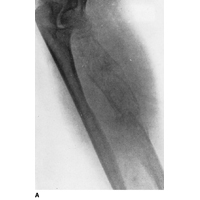 |
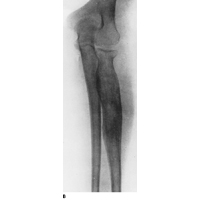 |
|
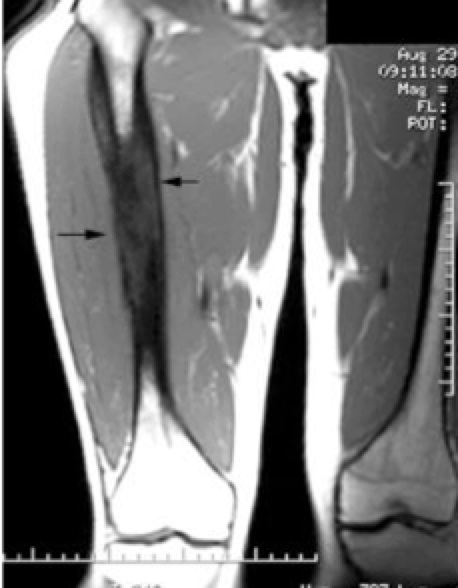 | 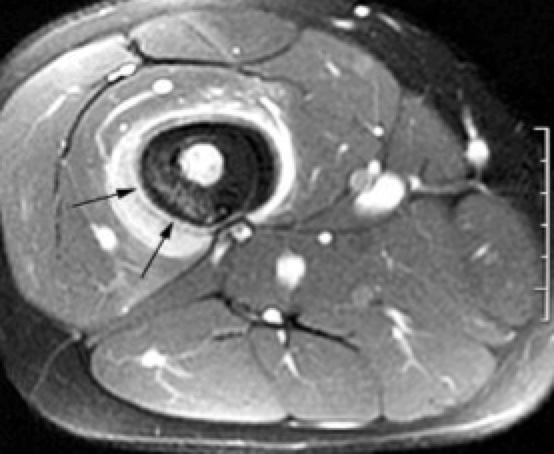 | Axial image shows bright enhancing sarcoma |
The frequncy of overt metastases is 25% - 30% for pelvic primaries and less than 10% for ribs and extremeties. Most of the mets are to lung (40%) and bones (40%). Less commonly, bone marrow, lymph nodes, soft tissue, visceral, or rarely CNS involvement are seen.
General Management and Treatment
Ewings Sarcoma is a systemic disease. Most paitnets have micrometastatic disease at diagnosis. Historically, local control alone only cures about 10%.
The treatment paradigm: Neoadjuvant chemotherapy → local therapy → consolidation chemotherapy.
The question of local therapy has not been addressed in head to head trials comparing surgery to radiation and selection bias confounds the data that is available. Local failure after RT alone is 20% or higher, but radiation is more commonly used in central and larger tumors with poorer prognosis. Some data favors surgery due to inadequate radiation doses and laock of QA in RT planning.
There are other factors to consider with radiation: secondary malignancy, functional and psychological deficits from therapies.
The German CESS study group has randomized trials. When an early trial demonstrated inferior radiation control, a failure mode analysis demonstrated inadequate radiation volumes were used. A subsequent study CESS demonstrated that when radiation fields were subject to external review, in fact, radiation and surgical arms were equivalent. Surgery is still used, and should be used for small lesions in expendible bones (fibula, ribs, etc), because of the potential late sequalae of radiation. Historically radiation has been the treatment of choice in Ewings.
Disease response to radiation is slow on CT/MRI/bone scan. the significance of residual soft tissue abnormalities and persistent uptake on bone scans is unclear. Tumor size reflects local control. For tumors > 8 cm, local control is around 80%, while tumors < 8 cm are 90%. Many surgical patients include patients with lower disease volumes and risk (they're simply easier to operate on), potentially skewing results. Consideration of relative functional deficits from radiation, verses surgery should be an important consideration in making treatment recommendations. The capacity for rehabilitation, functional loss, and psycho-social adjustment in adolescents ust be considered.
Chemotherapy
Early studies established chemotherapy in the role of Ewings Sarcoma treatment. Multiagent chemotherapy (VACAdr = vincristine, actinomycin-D, cyclophosphamide, adriamycin) resulted in OS=50%-70%. Later studies (CCG/POG ) evaluated 6 agent chemotherapy in alternation, with the use of etoposide and ifosfamide alternating with VACAdr. This demonstrated better event free survival and overall survival in non-metastatic disease. In metastatic disease, no difference was shown. In fact, there has been little progress in metastatic Ewing's sarcoma over the years.
Radiation Therapy
Radiation therapy is one of the earliest and common treatments and has a substantial role in the treatment of Ewing's sarcoma except in small expendible bones where surgery can debulk with minimal functional and cosmetic defects. There continues to be controversy between radiation and surgery concerning the relative advantages and disadvantages of each modality and pre-op v. post-op radiation.
Radiation Therapy Treatment Planning And Techniques
Volumes
Volumes are critical to local control with radiation. The early CESS study did not use central review of volumes and had an inferior control. When central volume review was performed, the radiation arm results improved substantially. The importance of adequate treatment volume cannot be over-emphasized.
POG-8346 tested proper radiation field size. 179 patients (21% had distant mets) were treated with induction cyclophosphamide + adriamycin → local treatment (surgery or radiation with VAC during RT) → vincristine + actinomycin D. The radiation arm was randomized to whole bone with boost or IFRT:
- RT ARM I: whole bone to 39.6 Gy → boost 16.2 Gy
- RT ARM II: IFRT to 55.8 Gy.
- No difference was seen between the arms at 5 years (EFS=42%)
- If protocol volume was violated, local control was worse.1
1Halperin reports this as a LC rate of "only 45%" for volume protocol violations but does not identify the actual local control rate in this study when protocol was met. Retrospective reviews have indicated local control with radiation between 75% to 95%, suggesting that volume must be adequate for LC.
Current volume recommendations for volume mandate fused MRI/CT to identify the full tumor extent. The entire bony abnormalilty and soft tissue mass are included as identified at diagnosis, before any treatment. This is the GTV. The CTV is the initial (pre-treatment extent of disease) GTV + 2 cm margin to cover potential occult tumor. The PTV is added per institutional setup uncertainty, generally 5 mm to 1 cm.
Body cavity treatments may be modified to account for normal tissues displacing shrinking tumor. In body cavity disease, the GTV is constructed from the pre-operative imaging, but it may be modified to reduce the GTV when shrinkage allows normal tissue to move back into position. The entire original bony abnormality is always treated.
Post-operative treatment has not been adquately defined. Based on data supporting local volume for primary RT, treatment to the pre-operative tumor bed plus margin (2 cm), with reduced fields to gross residual disease is reasonable. Encompassing the surgical incision is important.
Radiation should be 3d CT/MRI fused planned. Improved imaging must be used to delineate GTV and OAR. IMRT is available and useful for sparing growth plates, when possible and when sparing will not compromise treatment. Circumferential irradiation of skin is important in avoiding potentially devastating lymphedema. Conventional APPA or oblique opposed fields may be adequate, either with or without wedges or compensation.
For Askin Tumors, pre-treatment chemotherapy is used prior to surgery. This will shrink the tumor allowing better resection. The post-chemotherapy volume in this case appears to be adequate for local control irradiation volumes. If resection is done prior to radiation, there is a risk of positive margins which will require greater margins, and possibly significantly larger fields volumes.
Volumes
Current preoperative Radiation therapy volume recommendations are smaller than previously, with good local control (90%).
- GTVp
- Prechemotherapy extent of disease. MRI is used to identfy the extent of bone and soft tissue disease.
- CTVp
- 1.0-1.5 cm margin on the GTVp. For large bulky mass extending into a body cavity with displacement of organs, volumes can be modified to account for the change in mass effect.
- CTV-Post OP
- Postoperative CTVs are less well defined but generally include the Pre-op tumor bed with margins. If there is gross residual disease, reduced field boost is reasonable.
- GTV2 (boost volume)
- unresected residual visible or palpable tumor by imaging or physical exam. This includes pre-treatment abnormalities in bone and gross tumro in soft tissue, post-chemotherapy.
- CTV2
- CTV2 = GTV2 + 1 cm margin
- PTV2
- The PTV is either 0.5 cm for IGRT treated disease, or 1.0 cm for non-IGRT treated disease.
Current RT Volume recommendations:
- Use MRI to identify the pre-chemo extent of disease.
- GTV is defined as the pre-chemo extent of disease.
- CTV = GTV + 1.0 - 1.5 cm margin. This can be modified for bulky tumors displacing normal tissues
- PTV = CTV + 0.5 cm (IGRT) to 1.0 cm (non-IGRT) expansion
- GTV2 = gross residual disease
- CTV2 = GTV2 + 1.0 cm margin
- PTV2 = CTV2 + 0.5 to 1.0 cm margin.
Dose
There are three options for local control: Surgery alone for R0 resections (negative microscopic margins), 50.4 Gy postoperatively if margins are < 5 mm, 55.8 Gy for definitive treatment. Margins have decreased from whole bone or muscle to 2 cm (from 5 cm). Local failure is about 10%
- Surgery alone for negative margins > 5mm
- Post-op RT for margins < 5 mm to 50.4 Gy
- Definitive RT to 55.8 Gy
- Margins have descreased from whole bone to 5 cm to now 2 cm (with 1 cm PTV expansion)
- Vertebral Body is held to 45 Gy to respect cord tolerance
Local failure is about 10%
- GTVp, CTVp, PTVp receive 45 Gy at 1.8 Gy/fraction (pre-chemo extent of disease)
- GTV2 is defined as the residual disease or palpable tumor by imaging and physical exam.
In summary, Initial pre-operative, pre-chemotherapy volume gets 45 Gy at 1.8 Gy/fraction → residual unresected disease (GTV2) is boosted with 10.8 Gy to a total of 55.8Gy.
For post-op treatment, 50.4 Gy for R1 disease, and 55.8 Gy for R2 disease.
Lung Metastases — Special Radiation Circumstance
Local irradiaiion of lung metastases as well as the primary site has been shown to improve outcomes. Bilateral lung irradiation was shown in the CESS study with doses between 12 and 21 Gy to increase the rate of resolution of lung mets. Hansen (UCSF) suggests whole lung RT at 1.5 Gy/fraction to 15 Gy may be used for extensive disease, or consider surgical management in cases of ≤ 4 lung mets.
OAR/Dose Limits
- Epiphyseal Plates: > 20 Gy prematurely closes the plates, resulting in short limb. Limb length distances of 6 cm can be fixed surgically or with prostheses
- Spare 1 - 2 cm strip of skin to prevent lymphedema beyound 20-30 Gy
- Cord tolerance dose is still 45 Gy.
Outcomes, Patterns of Failure, Prognostic Indicators
The primary prognostic indicators are:
- Favorable:
- good response to chemotherapy
- Unfavorable
- Mets at diagnosis
- Large tumor volume (> 200 ml)
- Pelvic or central location
Radiation therapy is highly effective with a local control rate of between 75% and 95% after primary radiation treatment to about 50 Gy. Surgery is an alternative with similar control when used in small, expendible bones. Treatment results with surgery and chemotherapy are excellent, too.
The German CESS (cooperative ewings sarcoma study) reported a significant difference in local control and survival favoring surgical approaches but this trial had an unexpectedly high rate of failure in the radiation arm attributed to inadequate radiation volume. The study did show equivalence with small, and presumably well irradiated lesions. CESS initiated a quality assurance program which demonstrated a remarkable improvement in radiation arms in subsequent trials.
The second CESS trial Randomized {I:chemotherapy → radical surgery; II: surgery + radiation to 45 Gy; III: radiation alone to 60 Gy } with central treatment planning review. The results are comparable to surgical groups with RFS3 of 67% (RT), 65% (Sx), 62% (SX+RT)