Medulloblastoma and Supratentorial PNET
Epidemiology
Adult Medulloblastomas are quite rare. The majority arise in the 20-40 year old group and the incidence rate is 0.5/100k (5/1M). Adult medulloblastomas are more frequently laterally than centrally located, and more frequently desmoplastic. Severe anaplasia is less in adults than the pediatric population.
Pathology and Natural History
Medulloblastomas are small round blue cell tumors with densely staining nuclei, frequent mitoses. Homer-Wright Rosettes are characteristic. CFS dissemination may manifest as positive cytology or macroscopic seeding of the sub-arachnoid space. CSF dissemination is not uncommon. Systemic spread is seen in about 5% of the population. Most manifestations of systemic spread are to bone and bone marrow. There is debate on the role of VP shunts in disseminating the disease.
Pediatric adverse indicators include male gender, and age < 3 years. Patients with total or near-total resections do better than those who have subtotal resection or biopsy only, with residual tumor > 1.5 cm2 on post-operative imaging. (i.e. the surgical goal is to get the residual disease to less than 1.5 cm2). Risk stratification classifications are used:
- Average risk: residual tumor < 1.5 cm and no dissemination
- High risk: residual tumor > and/or dissemination and/or large cell/anaplastic
Clinical Workup and Evaluation
Initial workup is imaging (after, of course the H&P), followed by surgery then the post-operative work up and staging. The surgical work up is maximum safe resection. If resection is unsafe, a stereotactic biopsy or sub-total resection should be performed to the extent feasible. A post-operative MRI should be obtained within 24-72 hours post-op. CSF should not be obtained before a spinal MRI clears the spinal cord, and should be delayed until 2-3 weeks post-operative to avoid false positives.
General Management and Treatment
The standard brain paradigm: Maximum safe surgery → Radiation
Medulloblastomas spread by CSF dissemination. CSI is indicated in both standard risk and high risk disease. The CSI dose is between 30 Gy and 36 Gy in standard risk (slightly reduced dose) and is 36 Gy in high risk disease. The primary site is boosted to 54 Gy - 55.8 Gy without chemotherapy. If chemotherapy is given during radiation the doses for standard risk disease are reduced to 23.4 Gy to the CSI and boost dose remains 54 Gy - 55.8 Gy. If chemotherapy is used vincristine is delivered during radiation followed by CDDP, cyclophosphamide and vincristine.
For high risk disease (> 1.5 cm3, anaplastic or disseminated) the CSI dose is 36 Gy and the boost dose is 54 - 55.8 Gy. Note that the boost dose in all cases is carried to 54 - 55.8 Gy. Chemotherapy consists of vincristine weekly during radiation followed by CDDP, vincristine and cyclophosphamide.
Radiation Therapy Treatment Planning And Techniques
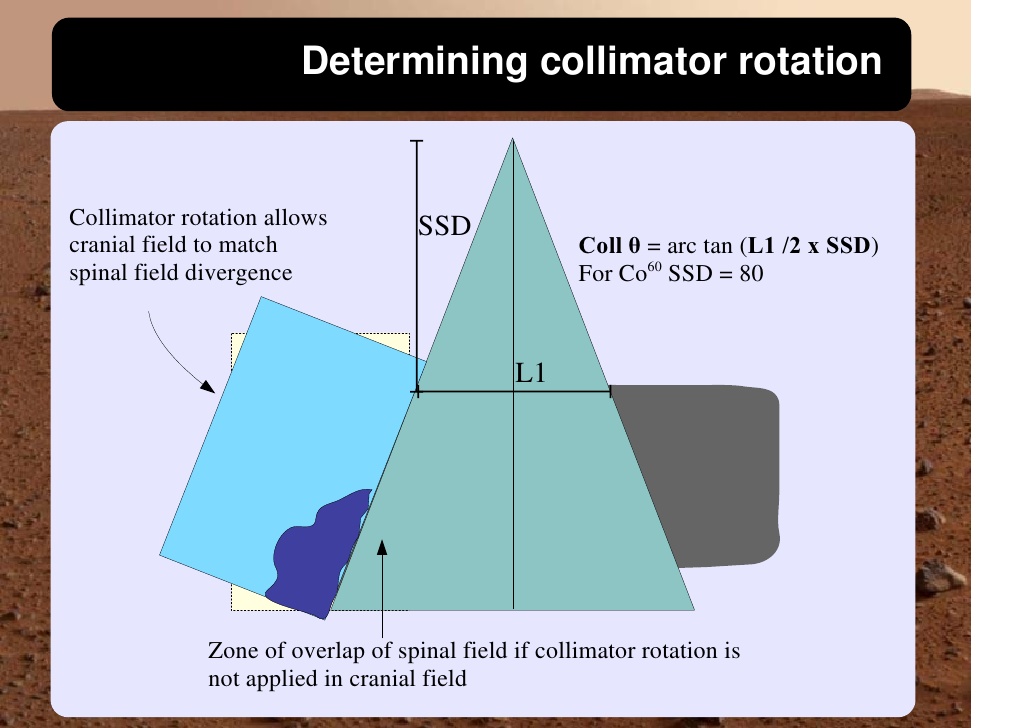
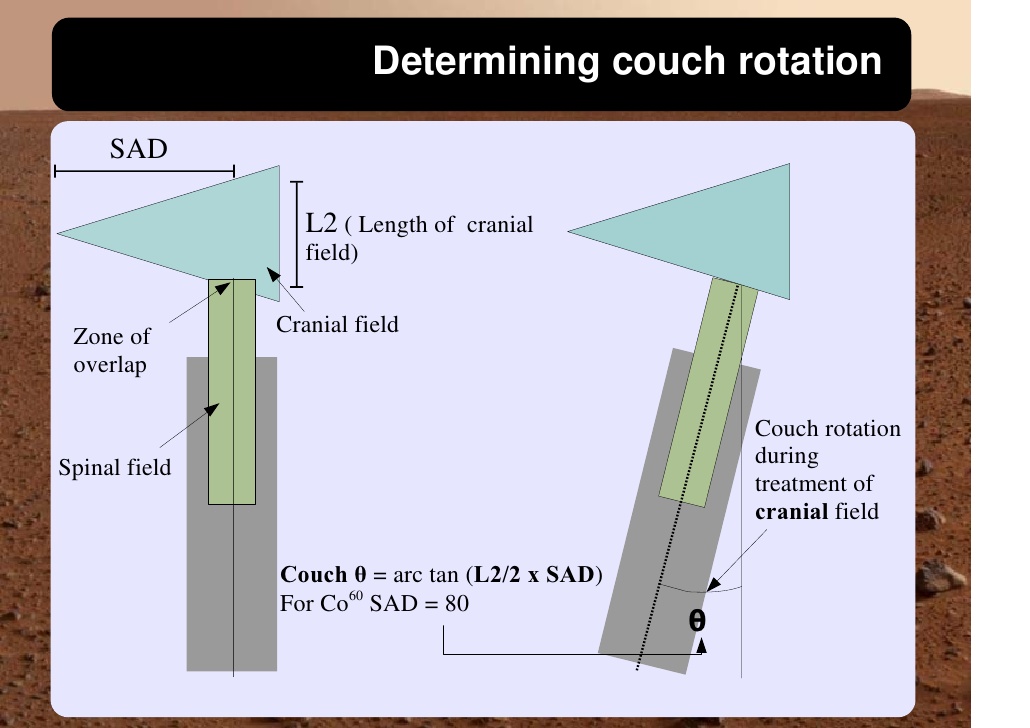
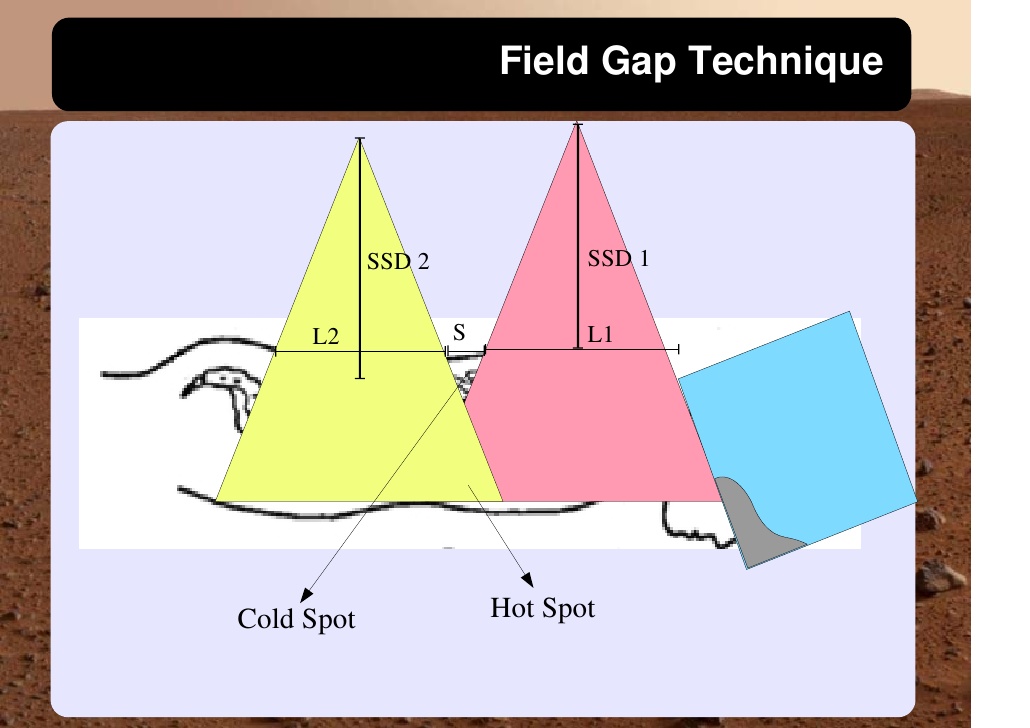
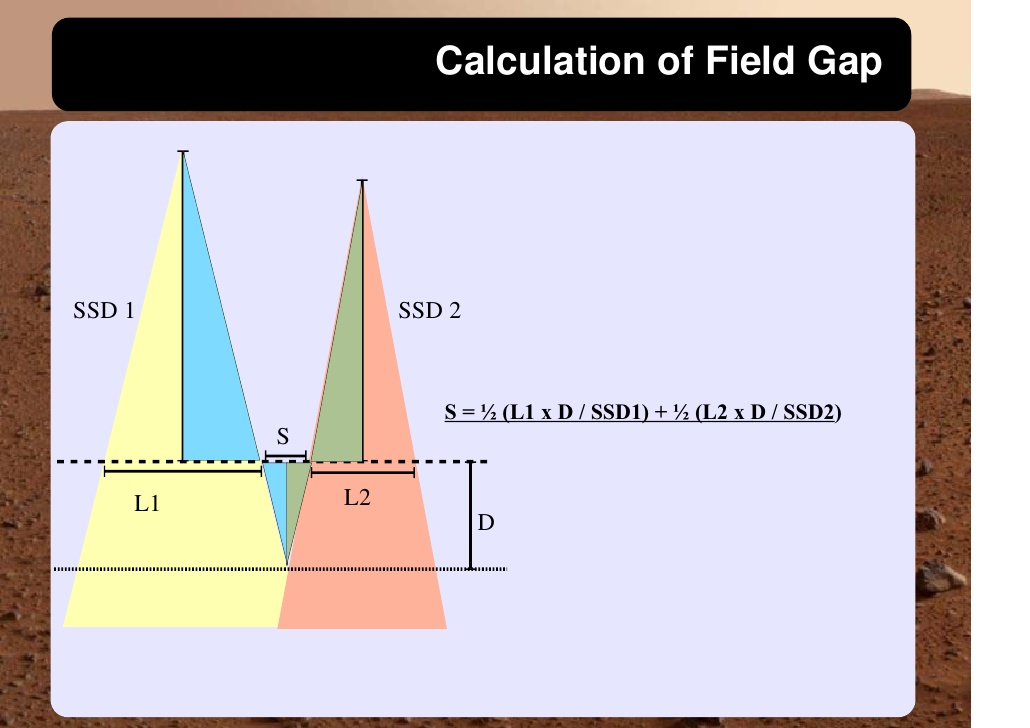
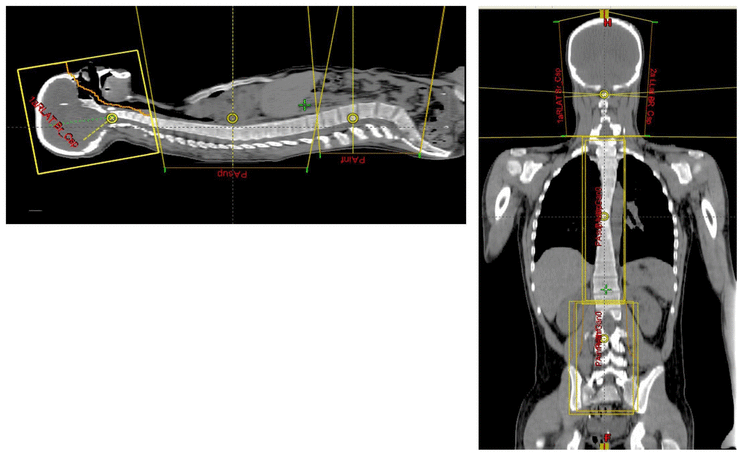
Cranio-spinal irradiation is technically challenging. There are a minimum of 3 and usually 4 beam divergence matches that must be accomplished. The brain is usually irradiated with opposed lateral fields, while the spine is irradiated with an orthogonal posterior-anterior field. The PA fields cover the spine portion. Key junctions are the upper PA field to the lateral fields, and the two PA fields. Traditionally the treatment fields have been set up with fluoroscopic simulators, which is time consuming and difficult. Several calculations are required. The following summarizes this type of technique. The patient is simulated prone with the neck extended. The match point of the upper PA and lateral fields is selected. This is usually selected in the cervical spine to minimize dose to larynx and thyroid. This also gave additional room to "feather the borders" to minimize the possibility of overdose/underdose due to setup uncertainty and variance. The setup junctions were feathered every 9 Gy (1/week) to insure uniformity of dose to the match lines. More modern techniques allow supine positioning with an aquaplast head mask, with or without trunk immobilization but with skin marks to determine straightness of setup match using room lasers. The basic principles are shown:
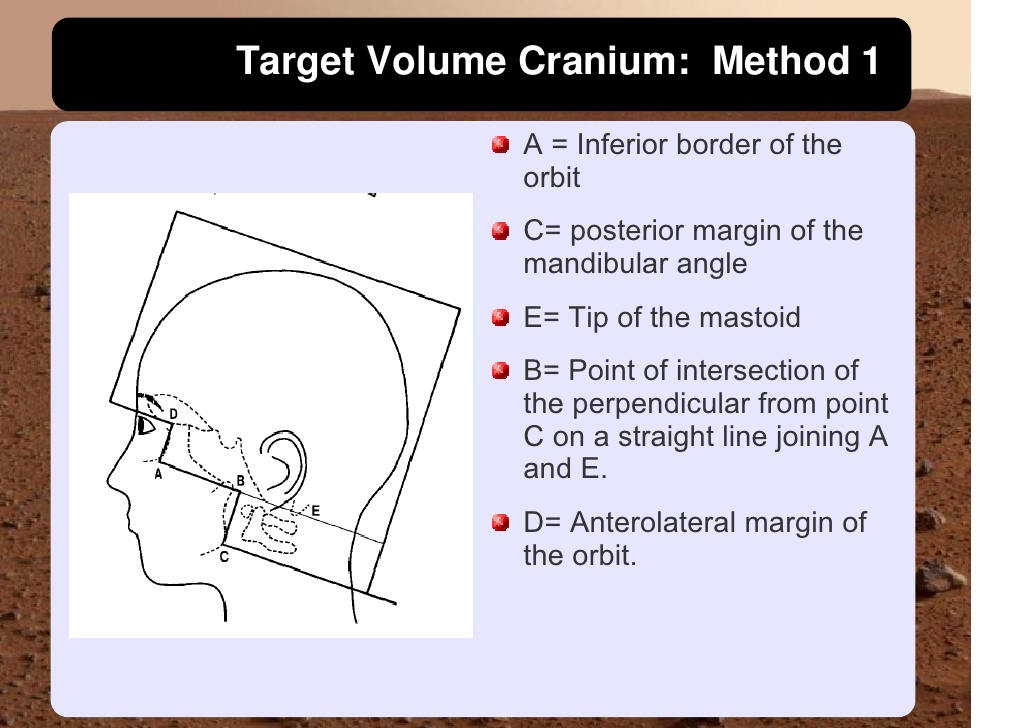 |
 |
 |
 |
 |
 |
 |
The general recommendations for treating medulloblastoma are:
Standard Risk disease: Residual disease < 5 cm3, no metastatic disease, localized, CSF negative, non-anaplastic/giant cell variants
- Maximum safe surgical resection
- Postoperative staging including MRI brain/primary post-op day 1-3
- Postoperative MRI spine at weeks 2-3
- Postoperative CSF tap if MRI spine is negative (POW 2-3)
- Standard Risk Disease (< 1.5 cm3 post-op residual, non-metastatic, CSF negative, non-anaplastic)
- Postoperative RT to begin within post-operative day 28-30 to the CSI:
- 23.4 Gy if concurrent vincristine → Vincr, CDDP, cyclophosphamide
- 30-36 Gy if no chemotherapy is contemplated.
- Identify and spare cochlea, if possible
- Boost Posterior fossa/GTV + 1 - 1.5 cm margin
- Postoperative RT to begin within post-operative day 28-30 to the CSI:
- High Risk Disease (≥ 1.5 cm3 residual, metastatic, anaplastic)
- Post-operative RT to begin within POD 28-30 to CSI
- CSI irradiation to 36 Gy (do not reduce the dose)
- Posterior fossa/GTV boost to 54 - 55.8 Gy
- For cord drop mets, treat 45 - 50 Gy.
Outcomes, Patterns of Failure, Prognostic Indicators
Adult medulloblastomas appear to have about the same prognosis as pediatric medulloblastomas. It is not clear if the biology is different. Post operative radiation therapy should commence within 28-30 days post resection whenever possible. Radiation therapy is initially delivered to the craniospinal axis followed by the involved site boost fields. The CSI is treated using opposed laterals to the posterior fossa with collimator rotated to match the superior PA field used for the spinal portion of the treatment. Great care must be used to match the field junctions. There are two competing divergences from each field. The lateral fields have divergence inferiorly into the superior PA spinal field, and the spinal field diverges superiorl into the lateral field. BOTH OF THESE DIVERGENCES MUST BE ACCOUNTED FOR AND CORRECTED. Once the PA field length in known, the collimator rotation of the lateral fields can be calculated (θ = atn(Len/2*SAD) )
Side Effects and Complications of Treatment
The side effects and complications of treatment are general CNS complications. An important risk that must be considered is the areas of dose in regions of overlap/underlap of abutting fields. This must be carefully controlled as described above. Cord dose limits must not exceed 50Gy, and bowel doses should not exceed 45 Gy, in areas of divergent overlap of beams. 3D conformal planning can help minimize this and gap edge feathering can help reduce these side effects. VISUALLY CONFIRM EACH SETUP FIELD, taking care to observe correct gantry rotation, table rotation and skin gap distances are correct, whether the patient is treated supine or prone.